Preparation method of nifuratel
A technology of nifuratel and molar ratio, applied in the field of preparation of nifuratel, can solve the problems of low utilization rate of reaction raw materials, high cost of raw materials, high production cost, etc., shorten reaction time, reduce production cost, and reduce dosage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 prepares methylthiomethyl vinyl carbonate
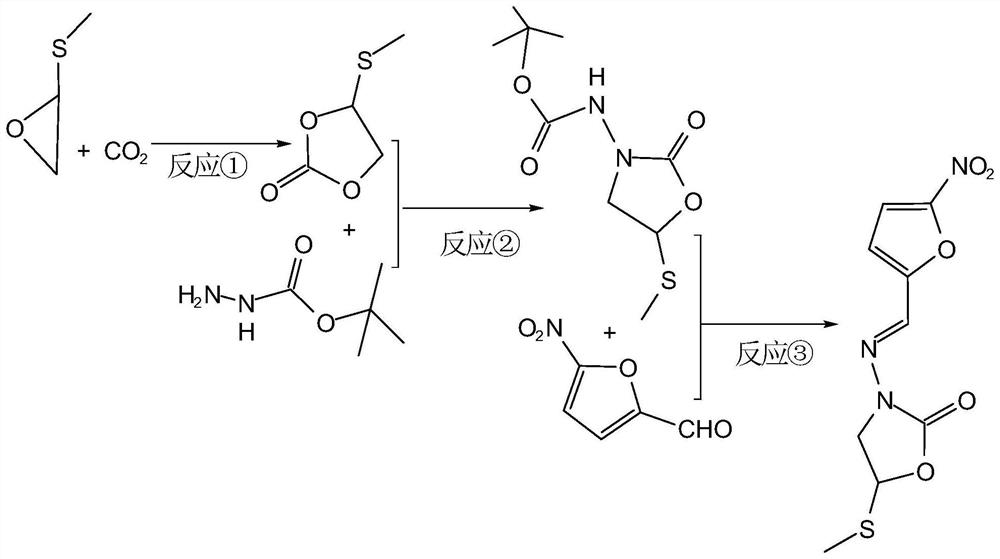
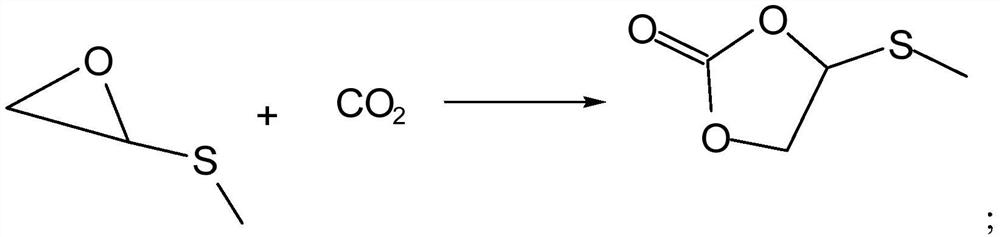
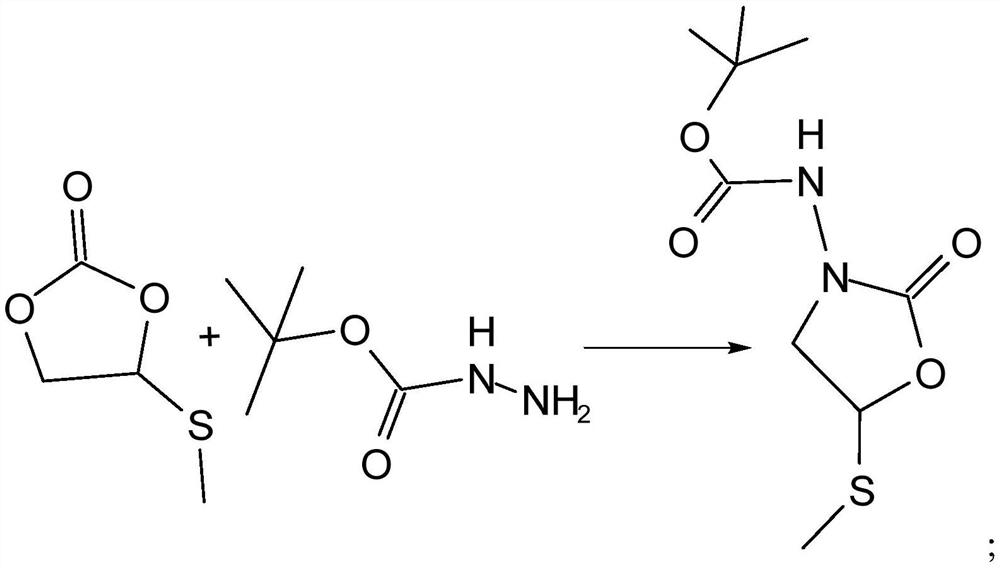
[0034] The specific steps are as follows: Add 0.1mol glycidyl methyl sulfide to a 1L autoclave, add 0.002mol 1-hydroxyethyl-1,8-diazabicyclo[5.4.0]undec-7- Alkenyl bromide catalyst, sealed, and then continuously filled with carbon dioxide gas, and maintained within a certain pressure, heated to 85 ° C under microwave treatment with a power of 1000W for addition reaction, the reaction time is 5 hours, the reaction process is as follows figure 1 As shown in the reaction ①, put it in an ice bath and cool it down to 6°C. During the liquid reaction process, the body partly solidifies, releases excess carbon dioxide, and removes the liquid to obtain methylthiomethylethylene carbonate. Use ethanol at 2 to 6 °C, dried at 30-35 °C, and weighed. The results are shown in Table 1.
[0035] Table 1
[0036]
[0037]
[0038] It is found from the above table 1 that as the pressure increases, the amount of methylthiometh...
Embodiment 2
[0039] Embodiment 2 prepares methylthiomethyl vinyl carbonate
[0040] The specific steps are as follows: Add 0.1mol glycidyl methyl sulfide to a 1L autoclave, add 0.002mol 1-hydroxyethyl-1,8-diazabicyclo[5.4.0]undec-7- Alkenyl bromide catalyst, sealed, then continuously filled with carbon dioxide gas, and maintained within 1.6MPa, heated at a certain temperature under microwave treatment with a power of 1000W, and carried out an addition reaction. The reaction time is 5h. The reaction process is as follows figure 1As shown in the reaction ①, put it in an ice bath and cool it down to 6°C. During the liquid reaction process, the body partly solidifies, releases excess carbon dioxide, and removes the liquid to obtain methylthiomethylethylene carbonate. Use ethanol at 2 to 6 °C, dried at 30-35 °C, and weighed. The results are shown in Table 2.
[0041] Table 2
[0042]
[0043] Find through above table 2, the amount of glycidyl methyl sulfide is constant, and along with the ...
Embodiment 3
[0044] Embodiment 3 prepares methylthiomethyl vinyl carbonate
[0045] The specific steps are as follows: Add 0.1mol glycidyl methyl sulfide to a 1L autoclave, add 0.002mol 1-hydroxyethyl-1,8-diazabicyclo[5.4.0]undec-7- Alkenyl bromide catalyst, sealed, then continuously filled with carbon dioxide gas, and maintained within 1.7MPa, heated to 95°C under microwave treatment with a power of 1000W for addition reaction, the reaction time is a period of time, the reaction process is as follows figure 1 As shown in the reaction ①, put it in an ice bath and cool it down to 6°C. During the liquid reaction process, the body partly solidifies, releases excess carbon dioxide, and removes the liquid to obtain methylthiomethylethylene carbonate. Use ethanol at 2 to 6 °C, dried at 30-35 °C, and weighed. The results are shown in Table 3.
[0046] table 3
[0047]
[0048] Find through above table 3, the amount of glycidyl methyl sulfide is certain, and as time prolongs, the amount of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com