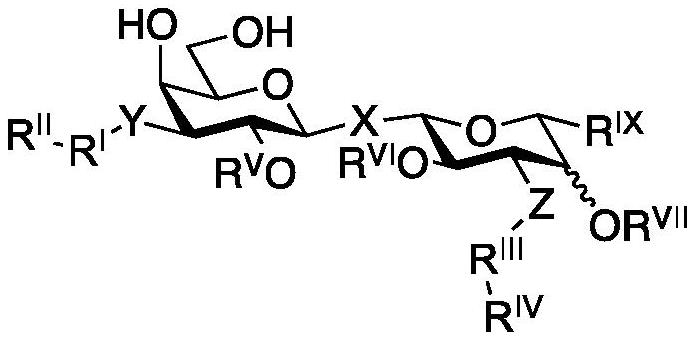
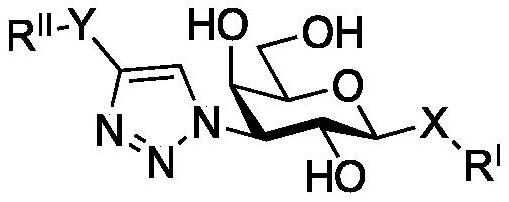
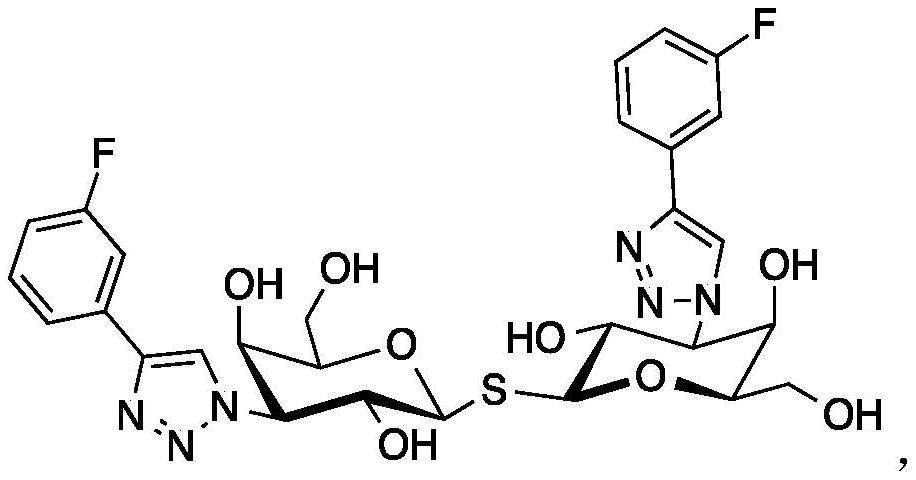
Novel galactoside inhibitors of galectin
A technology of galactopyranose and halogen, applied in the field of new compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 35
[0477] In vivo mouse pharmacokinetics of Example 35
[0478] Example 35 Intravenous (IV) administration of 1 mg / kg and oral (PO) administration of 10 mg / kg resulted in excellent systemic bioavailability, Figure 1 . Furthermore, good exposure was observed in the brain, Figure 2. After oral administration, the ratio of the exposure in the brain of Example 35 to the exposure in the plasma was determined as the AUC 脑 / AUC 血浆 = 0.19. (AUC = area under the curve)
[0479] Figure 1: Mean plasma levels after intravenous and oral administration
[0480]
[0481] Figure 2: Mean Brain Tissue Concentrations Following IV and Oral Administration
[0482]
[0483] Synthesis of Example Compounds and Intermediates
[0484] General experiment:
[0485] Nuclear magnetic resonance (NMR) spectra were recorded on a 400 MHz Varian instrument or on a 500 MHz Bruker Avance Neo 500 instrument at 25°C.
[0486] Chemical shifts are reported in ppm(d) using residual solvent as internal stand...
example 1
[0528] 3-Chlorophenyl-3-deoxy-3-[4-(3-fluorophenyl)-1H-1,2-pyrazol-1-yl]-1-thio-α-D-galactopyran Glycoside
[0529]
[0530]3-Chlorophenyl-3-(4-bromo-1H-1,2-pyrazol-1-yl)-3-deoxy-1-thio-α-D-galactopyranoside (30mg, 0.069 mmol), (3-fluorophenyl)boronic acid (19mg, 0.14mmol), K 2 CO 3 (38mg, 0.28mmol) and Pd(dppf)Cl 2 (7.6 mg, 0.010 mmol) in dioxane / water (1 mL, 2:1) was stirred at 60°C for 3 hours. The mixture was concentrated and analyzed by preparative HPLC (C 18 ,H 2 O / MeCN / 0.1% TFA) to give the title compound (5 mg, 16%). [C 21 h 20 ClFN 2 o 4 S][M+H] + ESI-MS m / z calculated for: 451.1; found: 450.7. 1 H NMR (400Mhz, methanol-d 4 )δ8.17(s,1H),7.92(s,1H),7.64(s,1H),7.55–7.51(m,1H),7.42–7.27(m,5H),6.93(t,J=7.8Hz ,1H),5.81(d,J=5.4Hz,1H),4.89–4.86(m,1H),4.57(dd,J=11.4,2.7Hz,1H),4.47(t,J=6.1Hz,1H) , 4.21 (d, J=1.9Hz, 1H), 3.75 (dd, J=11.4, 5.8Hz, 1H), 3.65 (dd, J=11.4, 6.6Hz, 1H).
example 2
[0532] 5-Chloropyridin-3-yl-3-[4-(4-chloro-3,5-difluorophenyl)-1H-1,2-pyrazol-1-yl]-3-deoxy-1-sulfur Di-alpha-D-galactopyranoside
[0533]
[0534] 5-chloropyridin-3-yl-3-(4-bromo-1H-1,2-pyrazol-1-yl)-3-deoxy-1-thio-α-D-galactopyranoside ( 49mg, 0.11mmol), (4-chloro-3,5-difluorophenyl) boric acid (43mg, 0.22mmol), K 2 CO 3 (78mg, 0.56mg) and Pd(dppf)Cl 2 (12mg, 0.017mmol) solution was dissolved in 1,4-dioxane / water (1mL, 2:1) and stirred at 90°C for 4 hours. The mixture was partitioned between EtOAc and water, the organic phase was dried and concentrated. The residue was passed through preparative HPLC (C 18 ,H 2 O / MeCN / 0.1% TFA) to give the title compound (5 mg, 9%). [C 20 h 17 Cl 2 f 2 N 3 o 4 S][M+H] + ESI-MS m / z calculated: 504.0; found: 503.8. 1 H NMR (400Mhz, methanol-d 4 )δ8.64(d, J=1.7Hz, 1H), 8.47(d, J=2.1Hz, 1H), 8.24(s, 1H), 8.20(t, J=2.0Hz, 1H), 7.97(s, 1H), 7.38(d, J=8.3Hz, 2H), 5.91(d, J=5.4Hz, 1H), 4.91–4.86(m, 1H), 4.61(dd, J=11.3, 2.7Hz, 1H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com