Dehydroabietic acid-based Schiff base multi-ion functional fluorescent probe as well as preparation method and application thereof
A technology for fluorescent probes and methyl dehydroabietate, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of high sample standard requirements and high professional operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
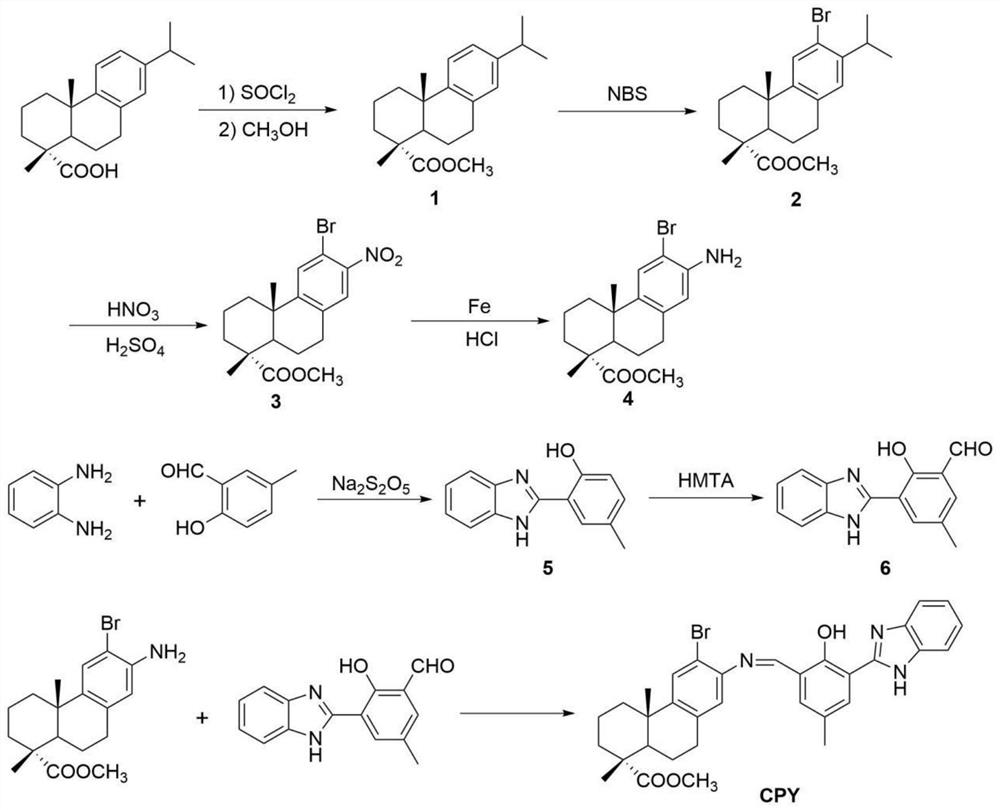
[0049] The present embodiment provides a method for preparing a dehydroabietate-based Schiff base multi-ion functional fluorescent probe, and the specific steps are as follows:
[0050] (1) preparation of 12-bromo-13-nitrodehydroabietic acid methyl ester: dehydroabietic acid is obtained through methyl esterification, bromination and nitration reaction, see figure 1 , the specific process is as follows:
[0051] Weigh 30 g of dehydroabietic acid and dissolve it in 60 mL of toluene, add 11 mL of thionyl chloride, react at 78-80 °C for 3 h, spin dry in vacuo, add 60 mL of methanol, react at 78-80 ° C for 3 h, spin dry, Add 30 mL of ethanol to dissolve the crystal to obtain methyl dehydroabietate;
[0052] Accurately weigh 5 g of methyl dehydroabietate, dissolve it in 30 mL of acetonitrile, add 4 g of N-bromosuccinimide, react at room temperature in the dark for 24 h, spin to evaporate, wash twice with dichloromethane, and dissolve the crystals in 100 mL of methanol to obtain Me...
Embodiment 2
[0062] Optimization of the Preparation Method of Dehydroabietate Schiff Base Derivatives:
[0063] Test 1: Accurately weigh 0.1 g of methyl 12-bromo-13-aminodeisopropyl dehydroabietate and 0.052 g of 3-(1H-benzo[d]imidazol-2-yl)-2-hydroxy-5 -methylbenzaldehyde, sonicated in 10 ml of absolute ethanol, the temperature was 78 ° C, refluxed and stirred for 12 hours, after the reaction was completed, placed at room temperature for recrystallization to obtain pure probe CPY with a yield of 72%.
[0064] Test 2: Accurately weigh 0.1 g of methyl 12-bromo-13-aminodeisopropyl dehydroabietate and 0.052 g of 3-(1H-benzo[d]imidazol-2-yl)-2-hydroxy-5 -methylbenzaldehyde, sonicated in 10ml of absolute ethanol, the temperature was 90°C, refluxed and stirred for 12 hours, after the reaction was completed, placed at room temperature for recrystallization to obtain pure probe CPY with a yield of 62%.
[0065] Test 3: Accurately weigh 0.1 g of methyl 12-bromo-13-aminodeisopropyl dehydroabietate ...
Embodiment 3
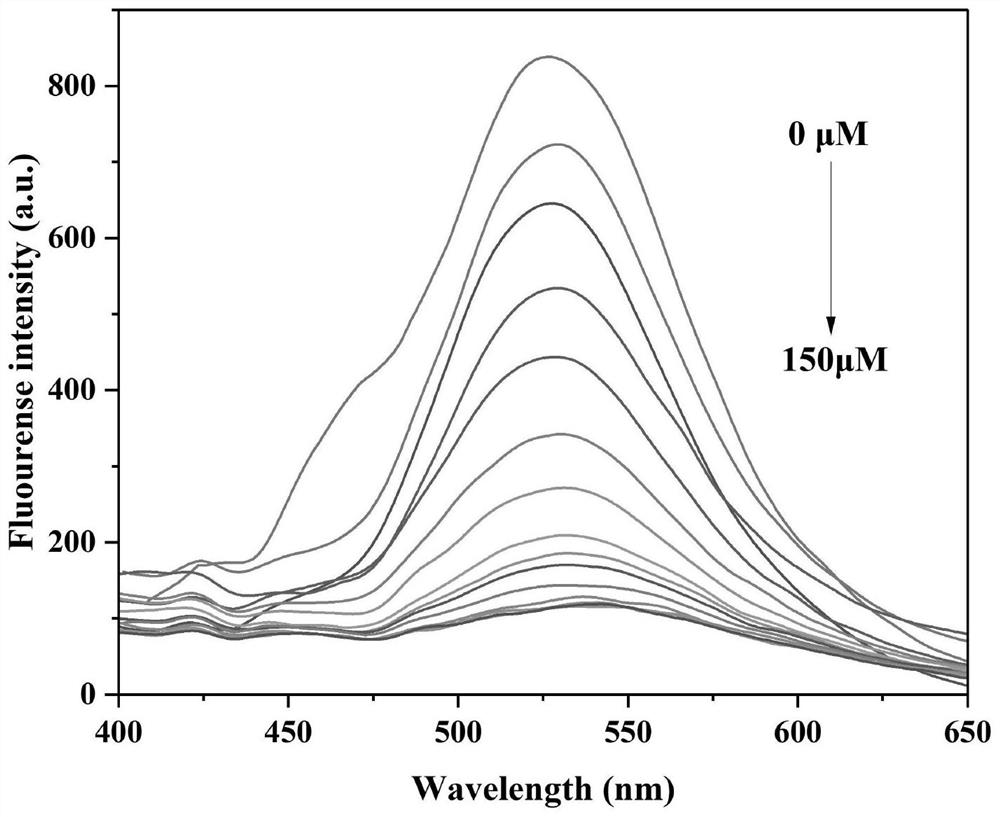
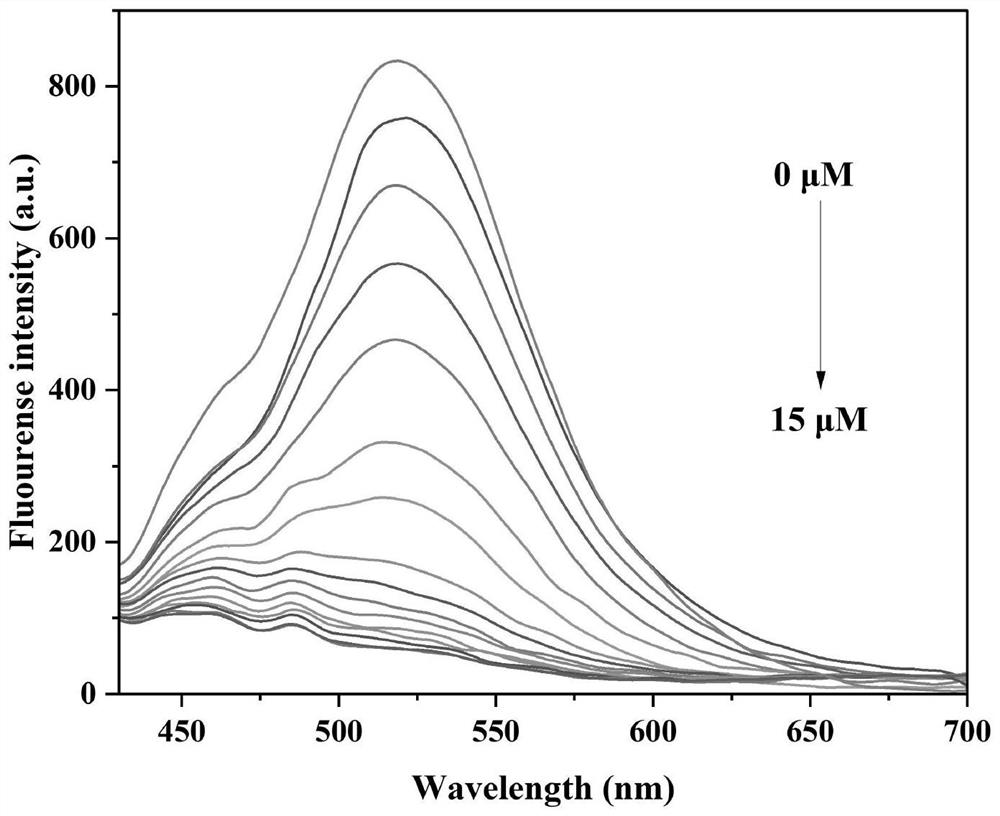
[0074] Dissolve the probe CPY in absolute ethanol to prepare 1 × 10 -3 M solution, take 200 μL of the stock solution and add it to 10 ml of ethanol aqueous solution (ethanol:water 7:3) to prepare 2×10 -5 M solution, add 5 μL Cu each time 2+ (1×10 -3 M), 50 μL ClO - (1×10 -3 M), 20 μL Hg 2+ (1×10 -2 M), the absorption spectra of ion-pair probe CPY with different concentrations were measured.
[0075] like figure 2 shown, adding Hg 2+ After that, with Hg 2+ With the increase of concentration, the green fluorescence at 520nm gradually weakened and gradually quenched, and the solution changed from yellow to reddish-brown under sunlight. It can be seen that at 0-150 μM Hg 2+ In the concentration range, the fluorescence intensity of compound CPY has a good linear correlation with the concentration of ions, the linear regression equation y=-5.32324x+829.08382, R 2 =0.98702, calculated as Hg 2+ The detection limit was 14.3 nM.
[0076] like image 3 shown, adding differ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com