Method and kit for detecting extremely acidic pH
A kit and acidic technology, applied in the field of a method and kit for detecting extremely acidic pH, can solve the problems of strong autofluorescence, short fluorescence signal emission wavelength, high scattering rate, etc., and achieve long fluorescence signal emission wavelength and extremely acidic pH The effect of sensitive response and low autofluorescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
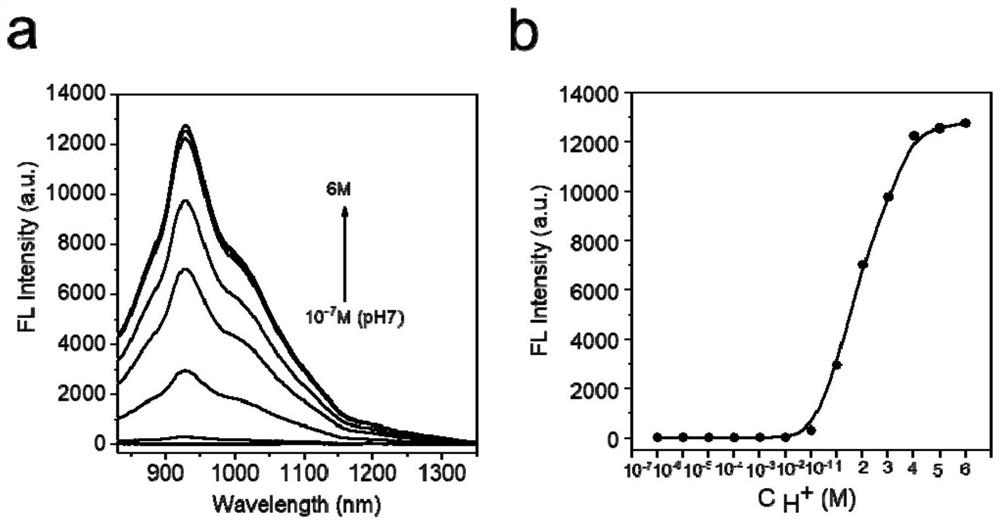
[0048] Example 1 Near-infrared second-region fluorescence emission spectra of protonated methylene blue (MBH) under different acidic conditions
[0049] First, use hydrochloric acid to configure the hydrogen ion concentration from 10 -7 A series of solutions from M to 6M, and then adding 50 μg / mL MB, will generate MBH immediately, and the reaction process is as follows figure 1 shown. Excited at 808nm wavelength, the near-infrared second region fluorescence emission spectrum was measured. figure 2 Shown are the near-infrared second region fluorescence emission spectra of MBH under different acidic conditions, figure 2 In a, the abscissa is the wavelength, and the ordinate is the fluorescence intensity, figure 2 In b, the abscissa is the hydrogen ion concentration, and the ordinate is the fluorescence intensity. figure 2 In a, it is shown that the fluorescence emission wavelength of MBH in the second near-infrared region under different acidic conditions is about 950 nm, ...
Embodiment 2A
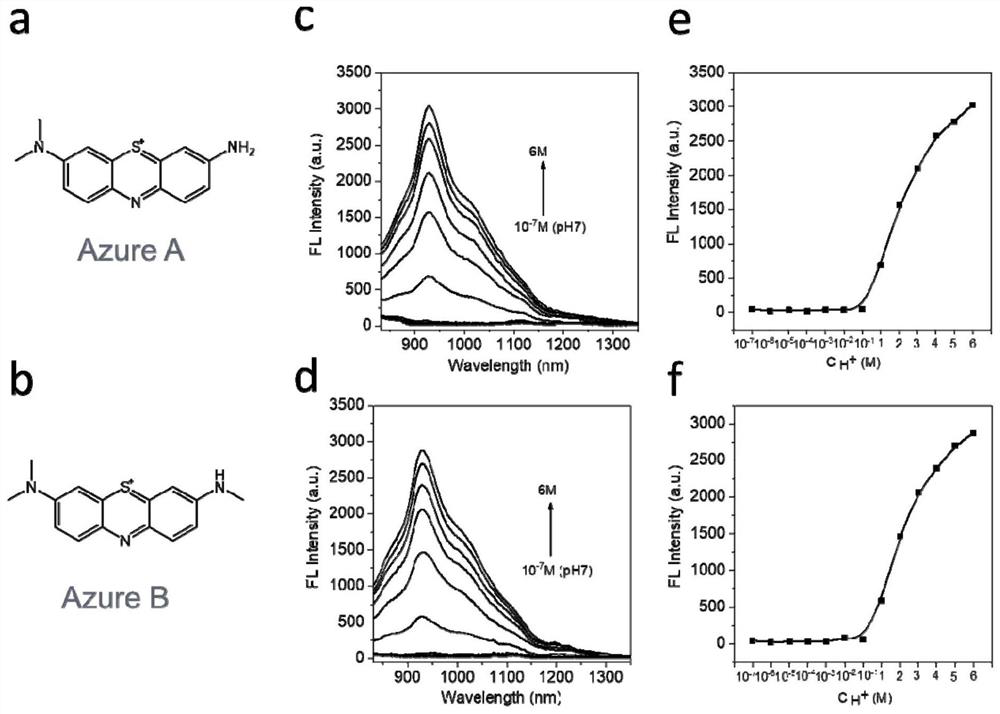
[0050] Example 2 Azure A and Azure B also have extremely acidic responsive near-infrared second region fluorescence emission properties
[0051] The present inventors also found that MB analogs Azure A (CAS number: 531-53-3, CAS name: 3-Amino-7-(dimethylamino)phenothiazin-5-ium chloride) and Azure B (CAS number: 531-55-5) , CAS name: 3-(Dimethylamino)-7-(methylamino)phenothiazin-5-iumchloride), they also have near-infrared second-region fluorescence emission properties in the environment of pH less than 1, such as image 3 shown, image 3 a and 3b are the structural formulas of Azure A and Azure B, respectively, image 3 c and image 3 d are the near-infrared second region fluorescence emission spectra of Azure A and Azure B under acidic conditions, respectively, image 3 e and image 3 f are the fluorescence intensities of Azure A and Azure B under different hydrogen ion concentrations, respectively. image 3 c and image 3 In d, the abscissa is the wavelength, and the ...
Embodiment 3
[0052] Example 3 The kit of the present invention is used to detect the pH value of the in vitro system
[0053] First, dissolve the phenothiazine salt (which can be any of methylene blue, Azure A and Azure B) in the kit with the solvent in the kit (which can be any of water, dimethyl sulfoxide, ethanol, and tetrahydrofuran). ), configured into a 50-500 μg / mL phenothiazine salt solution. Mix the aqueous solution with the system to be detected, and keep the final concentration of the phenothiazine salt at 50 μg / mL after mixing. After 1 minute, put the mixed system into a fluorescent dish, and use an Edinburgh FLS920 fluorescence spectrometer to detect the fluorescence spectrum. The wavelength is 808nm, the fluorescence signal is collected in the 900-1700nm band, and the intensity of the fluorescence signal is compared with the standard curve (the standard curve of methylene blue is figure 2 b, the standard curve of Azure A is image 3 e, the standard curve of Azure B is im...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com