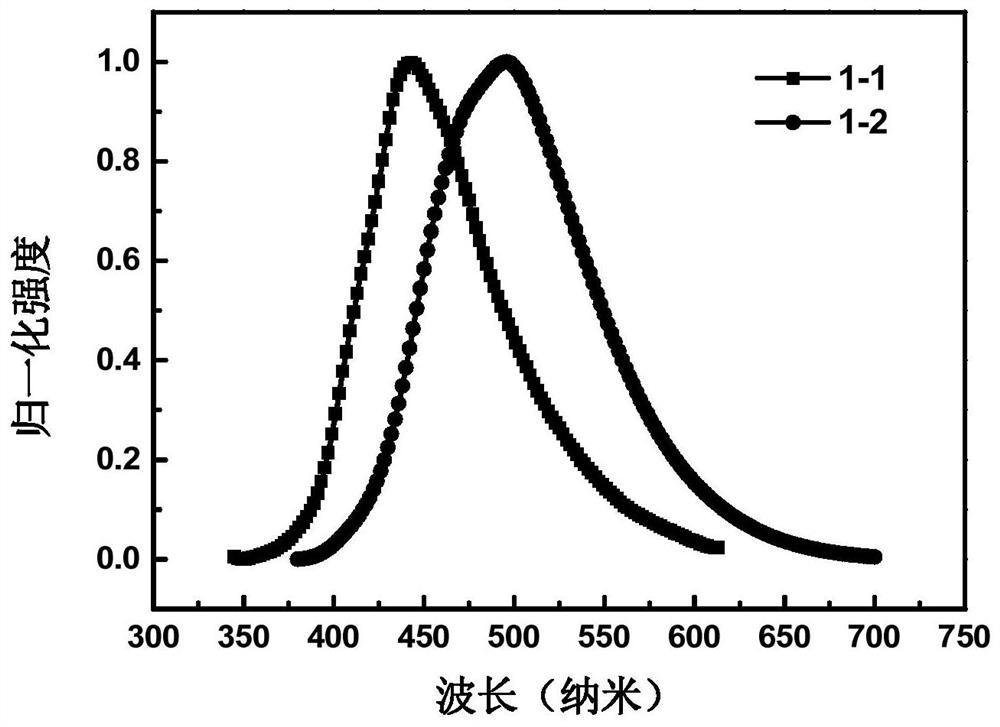
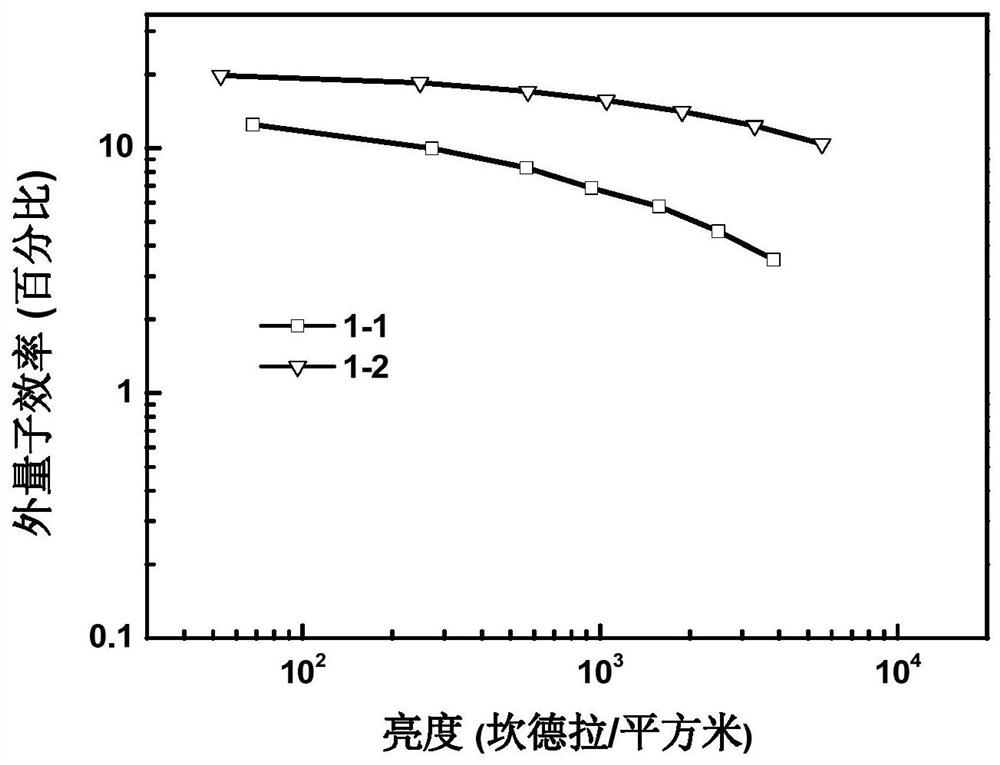
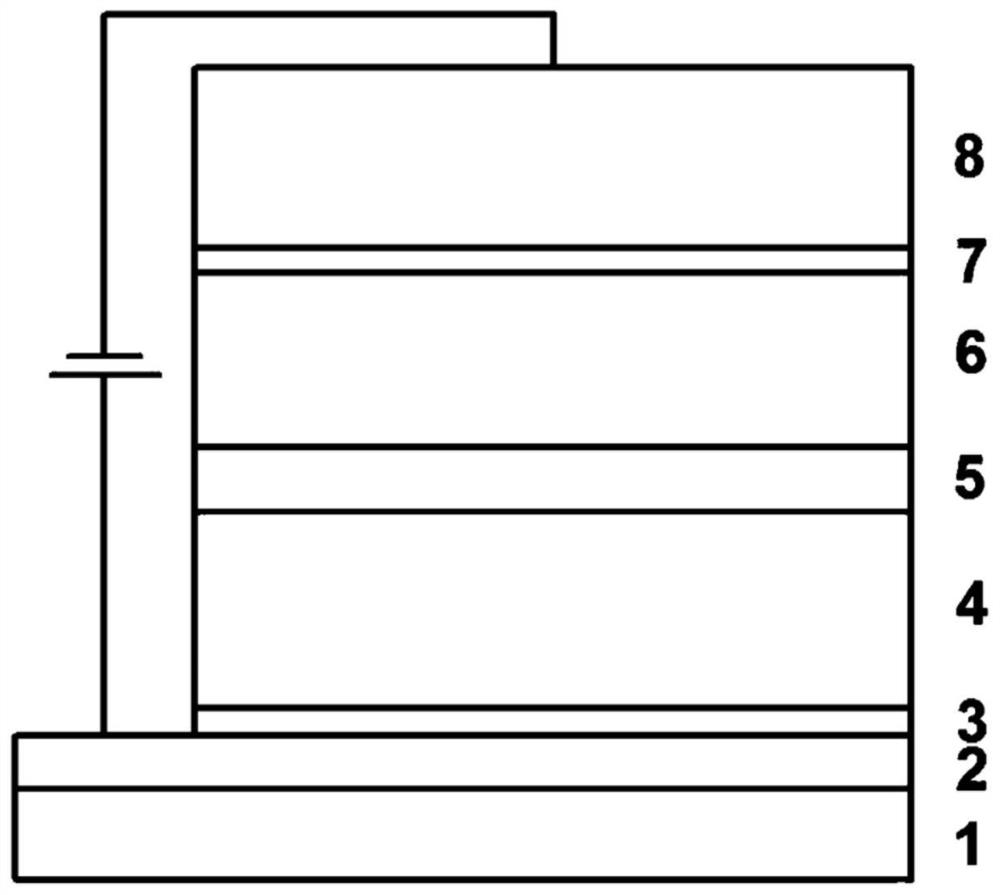
Phenylcarbazole spiro derivative and application thereof, organic electronic device, display device or lighting device
An organic electronic device, a technology of phenylcarbazole spiro, which is applied in the field of organic optoelectronic materials, can solve the problems affecting the application of organic electrophosphorescent devices, the efficiency roll-off of phosphorescent devices, and the reduction of external quantum efficiency, so as to improve device performance or efficiency. , Improved efficiency roll-off, excellent film formation and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Synthesis of Compound 1-1
[0032] The structural formula and synthetic route of compound 1-1 are shown in the following figure:
[0033]
[0034] The preparation method of the compound of formula M1 is as follows: 2.8g (16.8mmol) of carbazole, 11.6g (67.2mmol) of 1-bromo-2-fluorobenzene, 10.9g (33.5mmol) of cesium carbonate and 120mL of N are successively added to a 250mL two-necked flask, N-dimethylformamide was heated to 150°C with stirring for 24 hours. After the reaction was completed, the system was cooled to room temperature, poured into water, filtered under reduced pressure, and the filter residue was washed with a large amount of water. After separation and purification, 4.9 g of M1 was obtained with a yield of 90.8%. MS (ET): m / z 321.05 [M+]. Elemental analysis calculated value C 18 H 12 BrN (%): C, 67.10; H, 3.75; N, 4.35; found: C, 67.05; H, 3.70; N, 4.25.
[0035] The preparation method of compound 1-1 is as follows: under nitrogen prot...
Embodiment 2
[0036] Example 2: Synthesis of Compound 1-2
[0037] The structural formula and synthetic route of compound 1-2 are shown in the following figure:
[0038]
[0039] The preparation method of the compound of formula M2 is as follows: 2.3g (5.5mmol) 3,6-diiodocarbazole, 3.8g (22.0mmol) 1-bromo-2-fluorobenzene, 3.6g (11.0mmol) 3.6g (11.0mmol) are successively added to a 250mL two-necked flask ) Cesium carbonate and 120 mL of N,N-dimethylformamide were stirred and heated to 150° C. to react for 24 hours. After the reaction was completed, the system was cooled to room temperature, poured into water, filtered under reduced pressure, and the filter residue was washed with a large amount of water. After separation and purification, 2.9 g of M2 was obtained with a yield of 92.0%. MS (EI): m / z 572.75 [M+]. Elemental analysis calculated value C 18 H 10 BrI 2N (%): C, 37.67; H, 1.76; N, 2.44; found: C, 37.60; H, 1.75; N, 2.40.
[0040] The preparation method of the compound of f...
Embodiment 3
[0042] Example 3: Synthesis of Compounds 1-3
[0043] The structural formulas and synthetic routes of compounds 1-3 are shown in the following figure:
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com