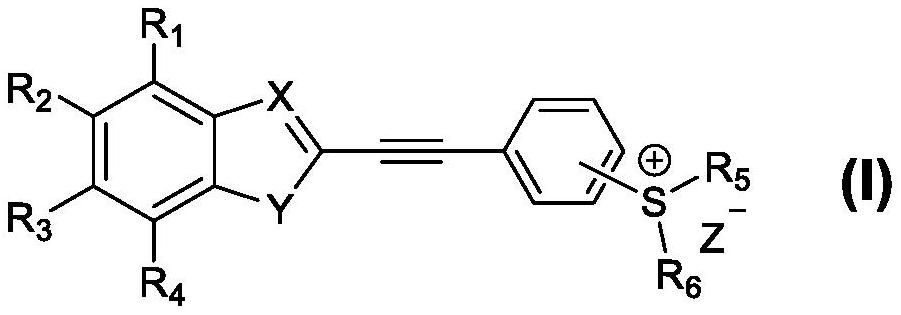
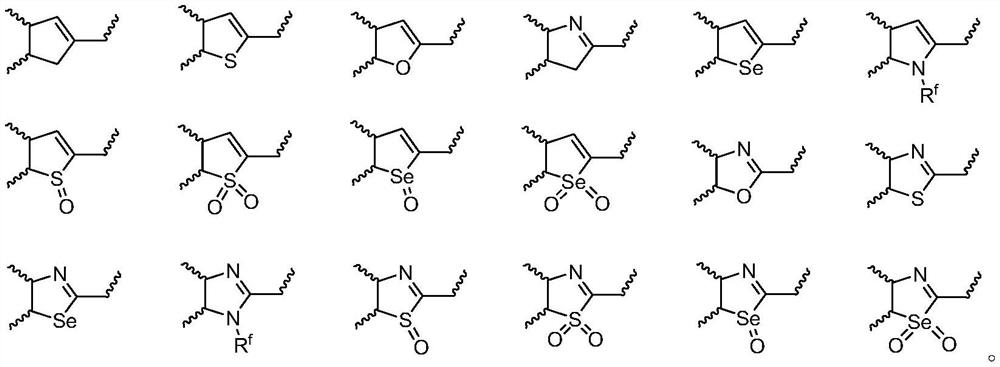
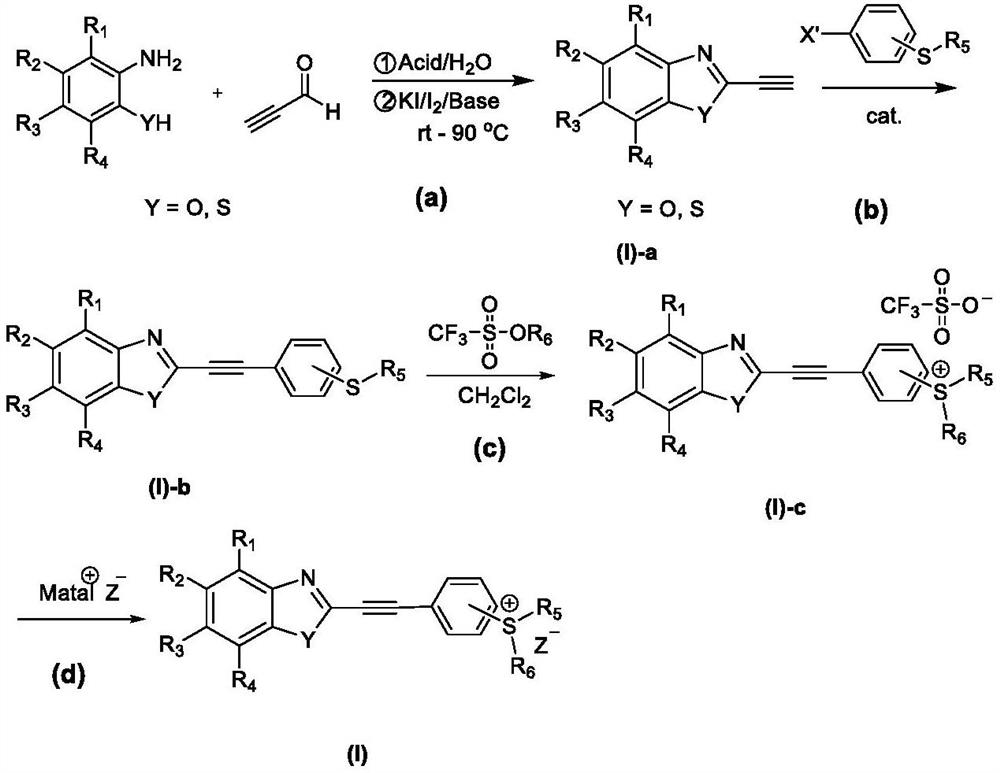
Benzo five-membered ring-benzyne sulfonium salt derivative as well as preparation method and application thereof
A five-membered ring and sulfonium salt technology, which is applied in the field of new material organic chemicals, can solve problems affecting the photoinitiation effect, achieve wide applicability, broaden the scope of application, and have good matching effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: Synthesize target sulfonium salt molecule (I)-1-PF according to the following route 6 -
[0051]
[0052] (a) Boric acid, water, room temperature, 3h; potassium iodide, anhydrous potassium carbonate, iodine, water, tetrahydrofuran, 50℃, 12h;
[0053] (b) bis(triphenylphosphine)palladium(II) chloride, potassium carbonate, N,N-diformamide, 130°C, 48h;
[0054] (c) methyl trifluoromethanesulfonate, dichloromethane, dark, room temperature, 24h; potassium hexafluorophosphate, room temperature.
[0055] 1. Synthesis of (I)-1a
[0056] 2-Aminophenol (21.8g, 0.20mol), boric acid (3.09g, 0.05mol) and solvent distilled water (100mL) were added to a 250mL three-necked flask containing a magnetic rotor, and then propyne was slowly added dropwise through a constant pressure dropping funnel Aldehyde (10.8 g, 0.2 mol) was added to the reaction system, and after the addition was completed, the mixture was stirred at room temperature for 3 h, and the reaction proces...
Embodiment 2
[0065] Embodiment 2: Synthesize target sulfonium salt molecule (I)-2-PF according to the following route 6 -
[0066]
[0067] (a) Boric acid, water, room temperature, 3h; potassium iodide, anhydrous potassium carbonate, iodine, water, tetrahydrofuran, 50℃, 12h;
[0068] (b) bis(triphenylphosphine)palladium(II) chloride, potassium carbonate, N,N-diformamide, 130°C, 48h;
[0069] (c) methyl trifluoromethanesulfonate, dichloromethane, dark, room temperature, 24h; potassium hexafluorophosphate, room temperature.
[0070] 1. Synthesis of (I)-2a
[0071] 2-aminothiophenol (25.0g, 0.20mol), boric acid (3.09g, 0.05mol) and solvent distilled water (100mL) were added to a 250mL three-necked flask containing a magnetic rotor, and then slowly added dropwise through a constant pressure dropping funnel Propargyl aldehyde (10.8 g, 0.2 mol) was added to the reaction system, and after the addition was completed, the mixture was stirred at room temperature for 3 h, and the reaction proc...
Embodiment 3
[0080] Embodiment 3: Synthesize target sulfonium salt molecule (I)-7-PF according to the following route 6 -
[0081]
[0082] (a) Boric acid, water, room temperature, 3h; potassium iodide, anhydrous potassium carbonate, iodine, water, tetrahydrofuran, 50℃, 12h;
[0083] (b) Copper powder, potassium carbonate, 18-crown-6, o-dichlorobenzene, 200℃, 48h;
[0084] (c) bis(triphenylphosphine)palladium(II) chloride, potassium carbonate, N,N-diformamide, 130°C, 48h;
[0085] (d) methyl trifluoromethanesulfonate, dichloromethane, dark, room temperature, 24h; potassium hexafluorophosphate, room temperature.
[0086] 1. Synthesis of (I)-7a
[0087] Add o-phenylenediamine (21.6g, 0.20mol), boric acid (3.09g, 0.05mol) and solvent distilled water (100mL) to a 250mL three-necked flask containing a magnetic rotor, then slowly add propargyl aldehyde dropwise through a constant pressure dropping funnel (10.8 g, 0.2 mol) was added to the reaction system, and after the addition was compl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com