Prokaryotic expression and purification method and application of PK34 antibacterial peptide
A purification method and prokaryotic expression technology, applied in the field of prokaryotic expression and purification of PK34 antimicrobial peptides, can solve the problems of long production cycle, high cost, increased treatment cost, etc., and achieve low production cost, short production cycle and high product quality. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A prokaryotic expression and purification method of PK34 antimicrobial peptide,
[0048] step one:
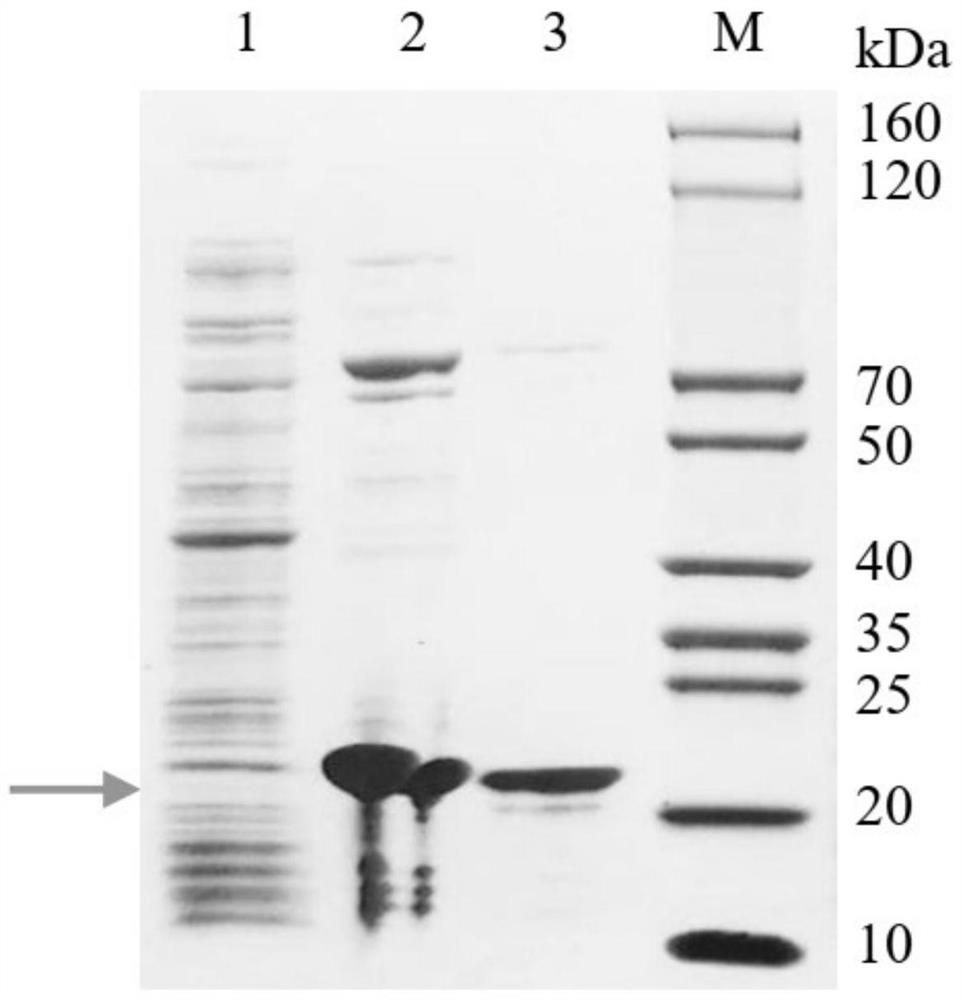
[0049] The codon optimization software MaxCodonTM Optimization Program (V13) optimizes the amino acid sequence of the provided PK34 protein, adopts the whole gene synthesis and inserts the PK34 gene into the expression vector pSumo-DT through the restriction enzyme sites StuI and HindIII, and through the enzyme Excision and sequencing confirmed the accuracy of the final expression vector. To facilitate purification, His-SUMO-tag was designed at the N-terminus of the recombinant polypeptide.
[0050] Step 2:
[0051] (1) Expression vector transformation and induced expression:
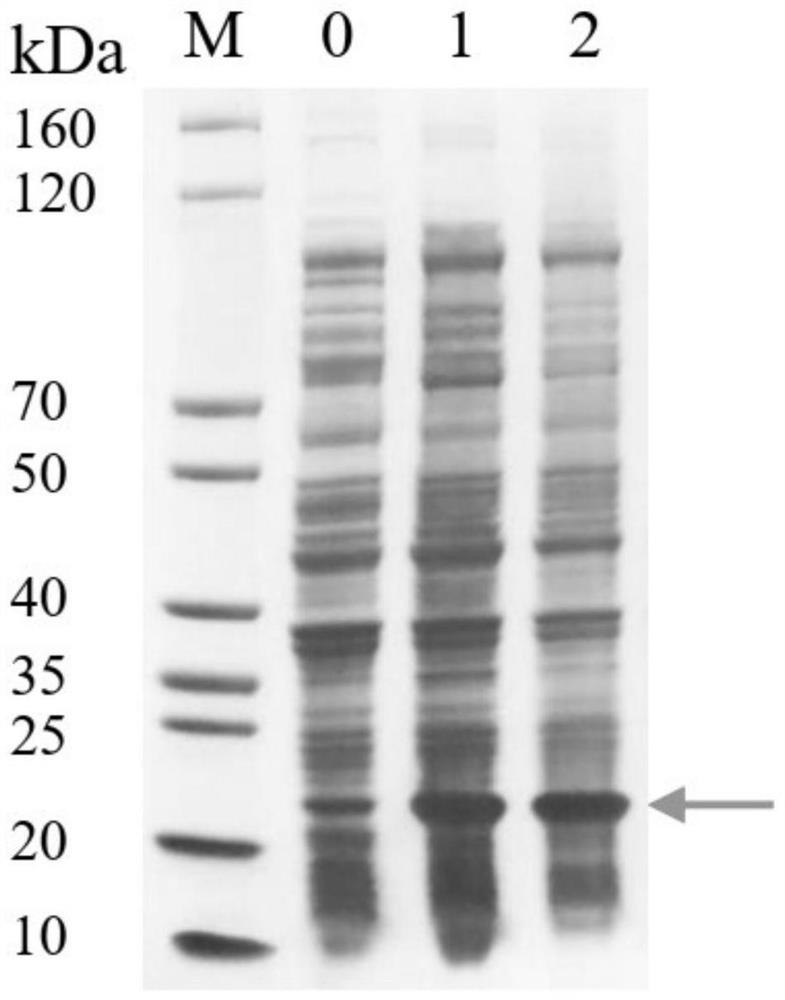
[0052]The constructed plasmid containing the PK34 gene was transformed into BL21 (DE3) competent cells, and then evenly spread on LB plates (containing 50 μg / mL kanamycin sulfate), and then placed in an incubator at 37°C overnight. Pick a single clone from the transformed plate, inoculate it i...
Embodiment 2
[0070] PK34 protein detection:
[0071] Step 1: PK34 protein stability test (freeze-thaw experiment):
[0072] Take a piece of PK34 protein frozen at -80°C, put it in an ice-water bath for 5-10min to slowly thaw, and then place it in a 4°C refrigerator for 0.5h after thawing. There is no abnormal phenomenon, indicating that the protein freeze-thaw experiment is normal.
[0073] Step 2: Determination of PK34 protein concentration:
[0074] The protein concentration was measured using a Bradford protein concentration assay kit, and the protein concentration was 0.4 mg / mL.
[0075] Step 3: Determination of PK34 protein purity:
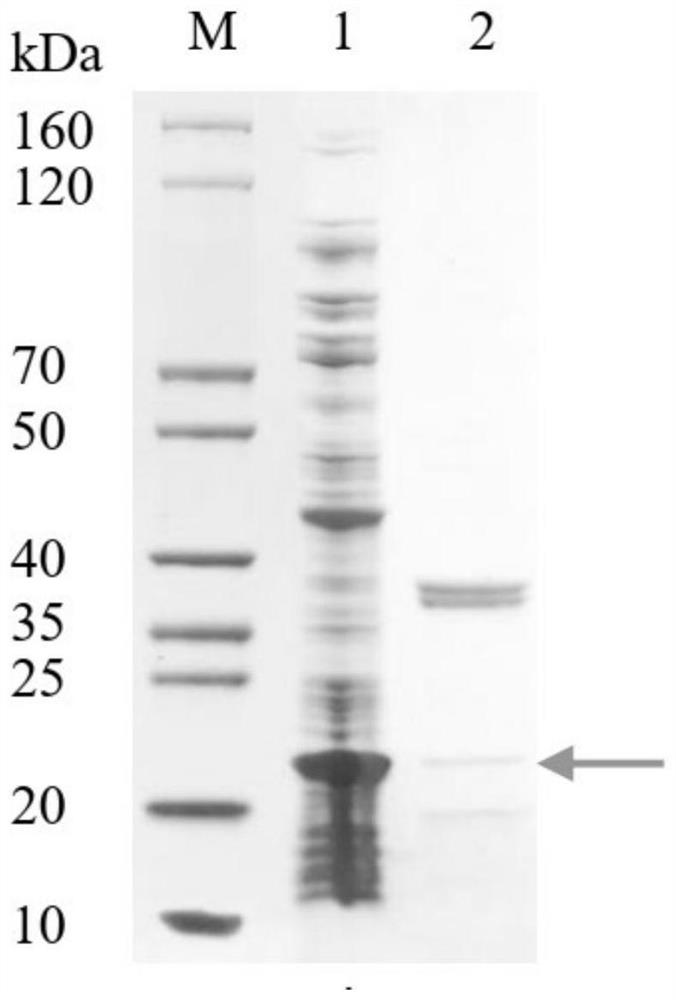
[0076] Determination of protein purity using SDS-PAGE method, BSA as the standard, purity > 85%, evaluation derived from R250 staining SDS-PAGE, such as Figure 5 Indicated, where Lane M: SDS-PAGE Marker; Lane 1: PK34 protein (1.50 μg); Lane 2: BSA (2.00 μg).
[0077] Step 4:
[0078] LC-MSMS protein identification:
[0079] (1) Enzymatic hydrolysis...
Embodiment 3
[0099] PK34 antibacterial effect verification:
[0100] The antibacterial effect detection method was a microdilution method based on resazurin redox colorimetry.
[0101] First, take a clean internal spiral glass tube, mix H37Rv in log phase with 7H9 medium containing 10% OADC, and adjust to 1 McFarland concentration (about ~10 7CFU / mL), note that the bacterial suspension should not contain bacterial clumps. Then mix the bacterial suspension with 7H9 medium containing 10% OADC at a ratio of 1:25, and dilute to about 5x10 5 CFU / mL bacterial suspension.
[0102] Set control group: ①Blank control group was 200uL 7H9 medium containing 10% OADC; ②positive control group was 100uL bacterial suspension+100uL 7H9 medium containing 10%OADC; ③negative control group was 100uL bacterial suspension+100uL 7H9 medium containing 10% OADC, and then 1uL of rifampicin stock solution (8mg / mL) was added to make the final concentration of rifampicin to be 40 ug / mL; ④ Resazurin blank control grou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Outer diameter | aaaaa | aaaaa |
| Inner diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com