Supported Ni/W-ZrO2 catalyst for hydrogen production by autothermal reforming of acetic acid
An autothermal reforming and catalyst technology, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, hydrogen and other directions, can solve the problems of poor thermal stability of catalyst structure, catalyst deactivation, etc. Effects of suppressing carbon deposition, promoting gasification, and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0024] Weigh 2.336g nickel nitrate hexahydrate and 6.381g zirconium oxynitrate dihydrate respectively, dissolve in water to form mixed nitric acid solution 1#; respectively weigh 2.212g ethylene glycol and 7.487g citric acid monohydrate, dissolve in water to form mixed solution 2 #; Mix 1# and 2# and heat in a water bath at 60°C, stir for 3-4 hours, until gel is formed, place it in a drying oven, and dry it at 105°C until the sample swells; put the dried The sample was placed in a tube furnace, heated from room temperature to 750°C at a rate of 10°C / min, and kept calcined for 4 hours to obtain a CDUT-NZ catalyst. The catalyst, in terms of the weight percent of oxides, was composed of: nickel oxide NiO 15.0%, zirconium dioxide ZrO 2 is 85.0%.
[0025] The evaluation of acetic acid autothermal reforming reaction activity was carried out in a continuous flow fixed bed reactor. The catalyst is ground and tableted, then sieved into 20-40 mesh particles, 0.1-0.2 g is weighed into ...
Embodiment 1
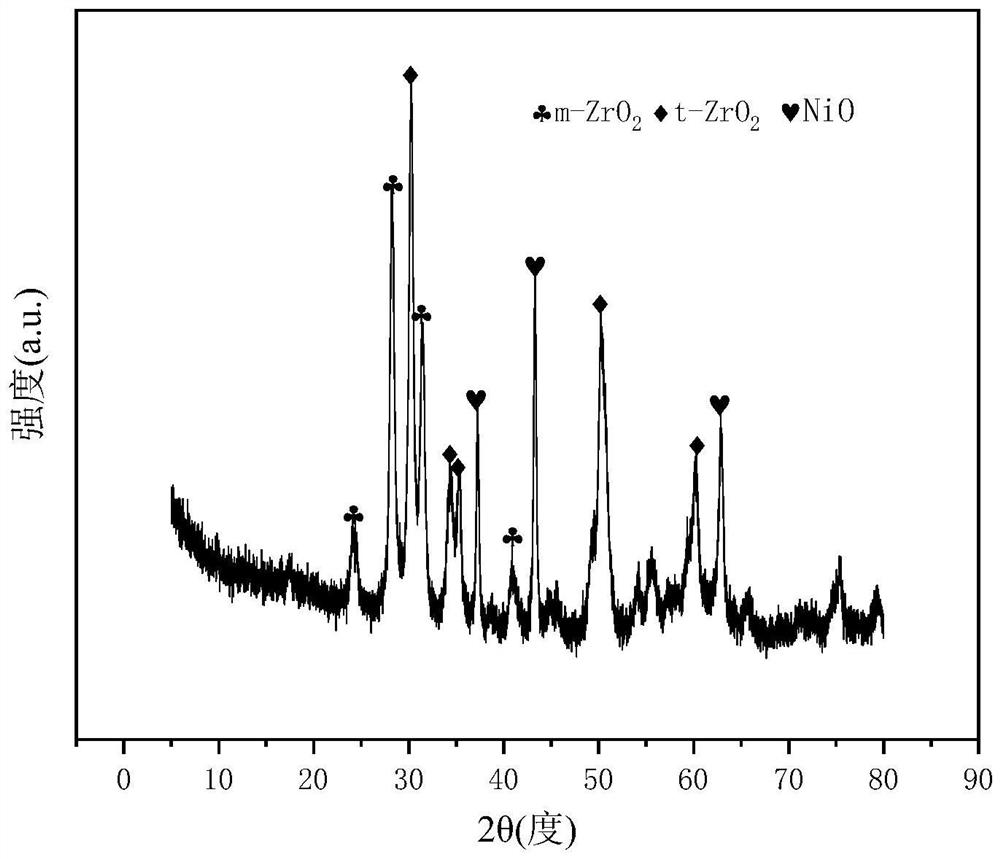
[0028] Weigh 2.336g nickel nitrate hexahydrate, 6.231g zirconium oxynitrate dihydrate and 1.024g ammonium tungstate respectively, dissolve in water to form mixed nitric acid solution 1#; respectively weigh 2.193g ethylene glycol and 7.423g citric acid monohydrate, dissolve Form mixed solution 2# in water; mix 1# and 2#, heat in a water bath at 60°C, stir for 3-4 hours, until gel is formed, place it in a drying box, and dry it at 105°C until The sample was expanded; the dried sample was placed in a tube furnace, heated from room temperature to 750 °C at a rate of 10 °C / min, and kept calcined for 4 hours to obtain the CDUT-NWZ-1 catalyst, which formed a catalyst supported on t-ZrO 2 / m-ZrO 2 Mesoporous Ni / W-ZrO with Ni-W-Zr-O Active Centers on Composite Phase 2 catalyst, whose typical crystal structure is shown in figure 1 shows that NiO is highly dispersed in t-ZrO 2 / m-ZrO 2 Compound ZrO 2 The crystal structure of the crystal phase forms the Ni-W-Zr-O active center; the t...
Embodiment 2
[0031] Weigh 2.337g nickel nitrate hexahydrate, 6.006g zirconium oxynitrate dihydrate and 2.548g ammonium tungstate respectively, dissolve in water to form mixed nitric acid solution 1#; respectively weigh 2.164g ethylene glycol and 7.328g citric acid monohydrate, dissolve Form mixed solution 2# in water; mix 1# and 2#, heat in a water bath at 60°C, stir for 3-4 hours, until gel is formed, place it in a drying box, and dry it at 105°C until The sample expanded; the dried sample was placed in a tube furnace, heated from room temperature to 750 °C at a rate of 10 °C / min, and kept calcined for 4 hours to obtain CDUT-NWZ-2 catalyst, which formed a catalyst supported on t-ZrO 2 / m-ZrO 2 Mesoporous Ni / W-ZrO with Ni-W-Zr-O Active Centers on Composite Phase 2 catalyst. The weight percentage of the catalyst in terms of oxides is as follows: nickel oxide NiO is 15.0%, zirconium oxide ZrO 2 80.0%, tungsten oxide WO 3 is 5.0%.
[0032] The catalyst CDUT-NWZ-2 was investigated for the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com