High performance liquid chromatography for determining N-nitrosodimethylamine in ranitidine and solid preparation thereof
A high-performance liquid chromatography and nitrosodimethylamine technology, which is applied in the field of high-performance liquid chromatography measurement of N-nitrosodimethylamine, can solve the problems of mass spectrometry detectors, increased inspection costs, and relatively low impact on measurement results. Large and other problems, to achieve the effect of simple maintenance cycle, long maintenance cycle and less adjustment of detection parameters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
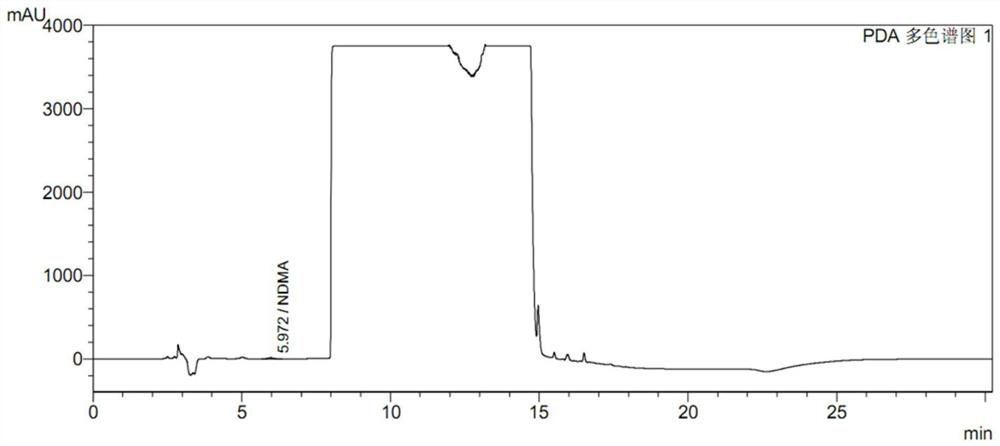
[0075] Take an appropriate amount of ranitidine or its solid preparation (about 1.5g of ranitidine), add 2.5ml of water precisely, dissolve it by ultrasonic for 2 minutes, precisely add 0.5ml of 0.35g / ml sodium hydroxide solution, shake well, and add 1g of precision 2ml / ml silver nitrate solution, shake well, accurately add 1ml of 0.2g / ml sodium chloride solution, shake well, stand for 10 minutes, centrifuge and filter. The filtrate should be clear and colorless. If it is turbid, it can be frozen at -20°C for 10 minutes, then centrifuged and filtered.
[0076] After repeated verification, under the above proposed operation, ranitidine hydrochloride is almost completely precipitated, and the test solution can change from a pale yellow viscous liquid before precipitation to a clear and non-viscous state. The filtrate of each test batch showed no turbidity after dropwise addition of sodium chloride solution, indicating that the silver ions were completely removed. After more tha...
Embodiment 2
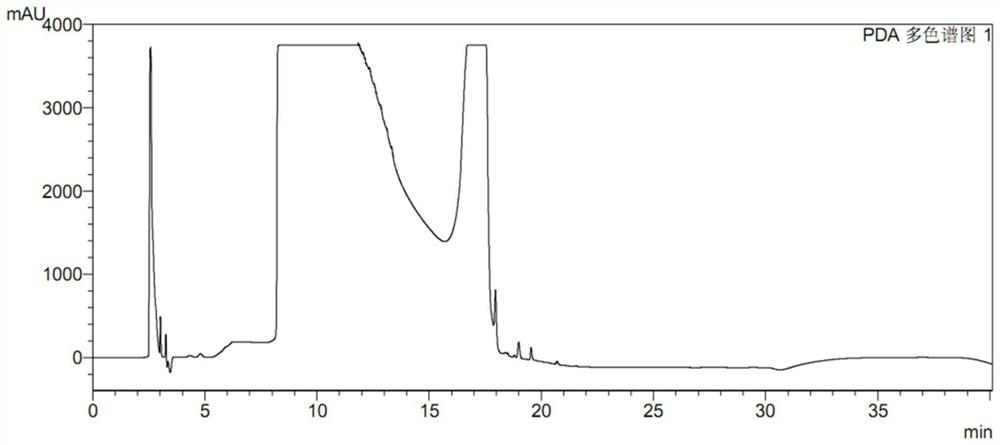
[0093] like Figure 8 As shown, collect the ranitidine hydrochloride capsules of new factory, deal with according to the embodiment of the present invention 1, detect the NDMA lower than the limit, its content determination result is consistent with the current mass spectrometry method detection result, again shows that the inventive method is effective to NDMA. The detection ability reaches the level of mass spectrometry.
Embodiment 3
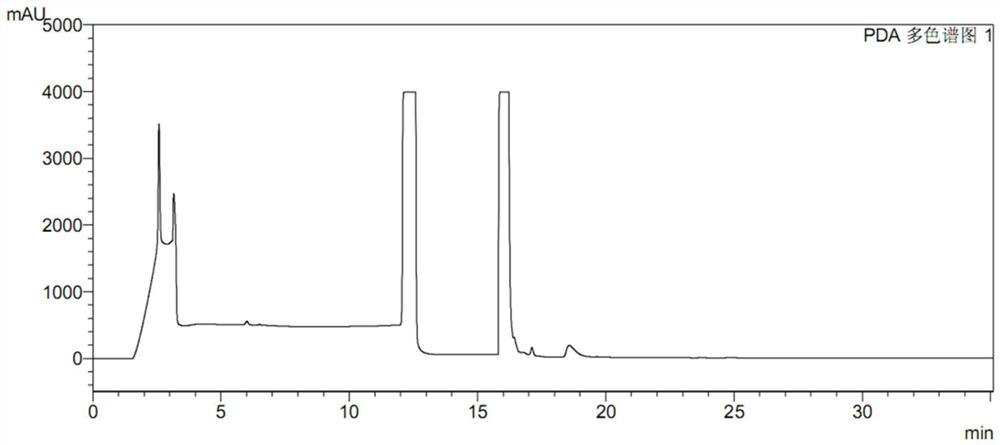
[0095] Take the same batch of newly manufactured ranitidine hydrochloride capsules and place them at 80°C for 6 hours. Figure 9 As shown, processing according to the present invention results in about 1000 times the detection limit of NDMA. It shows that the method has good specificity and high sensitivity, and also shows that the tendency of ranitidine to degrade in high temperature environment to generate NDMA is extremely obvious.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com