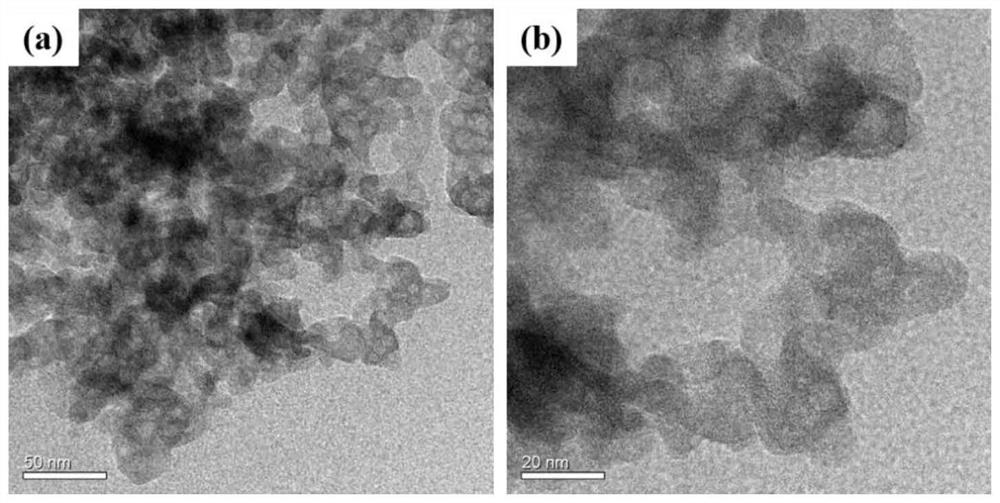
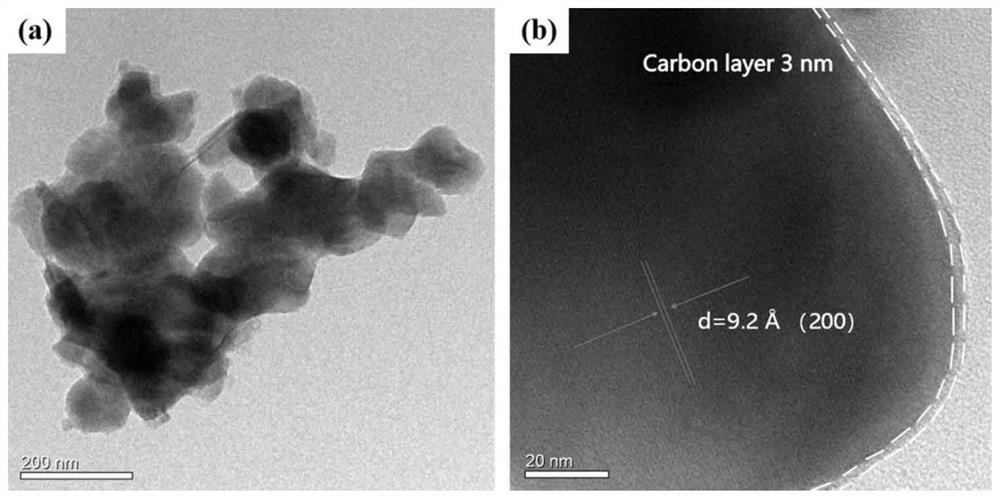
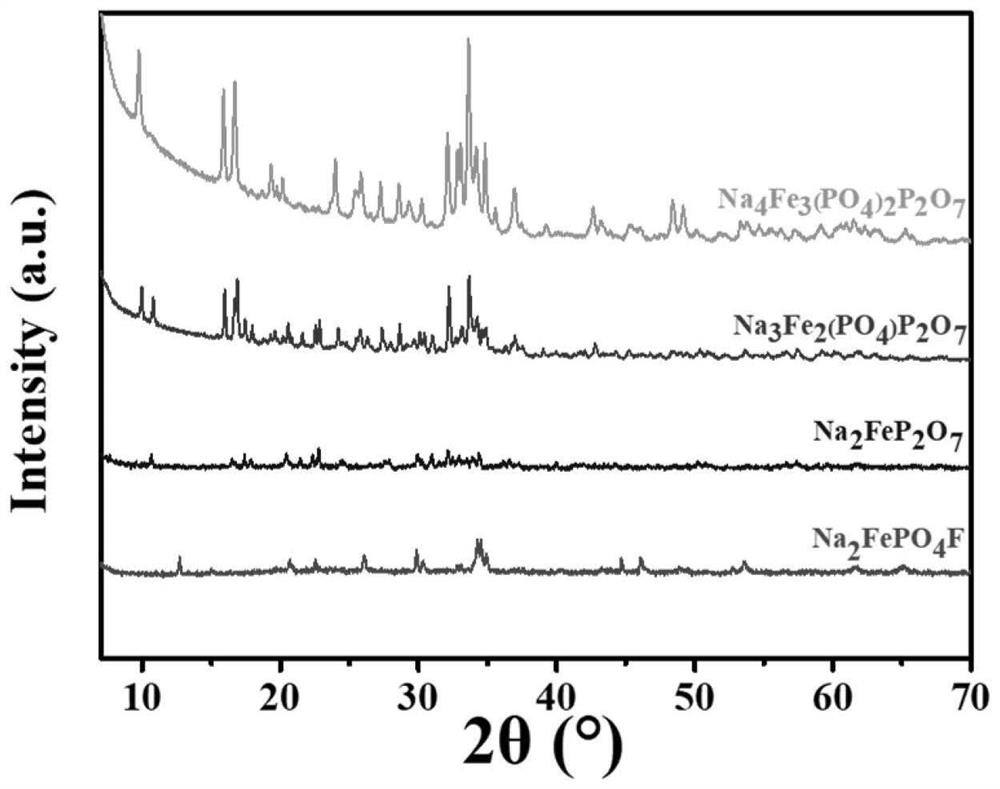
Iron-based phosphate sodium ion battery positive electrode material and preparation method thereof
A technology of iron-based sodium phosphate and sodium-based phosphate, applied in battery electrodes, electrode manufacturing, secondary batteries, etc., can solve problems such as side reactions and affecting cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of Na by sol-gel method combined with high temperature solid phase method 2 FeP 2 O7 .
[0035] 2.62 g NH 4 H 2 PO 4 and 1 ml of aniline were dissolved in 200 ml of deionized water, and 5.51 g Fe(NO) was slowly added dropwise with stirring. 3 ) 3 The aqueous solution obtained by dissolving in 100 ml of deionized water was stirred at room temperature for 5 h to obtain a polyaniline-coated iron phosphate precursor;
[0036] Then add 3.04 g Na to the mixed solution containing the iron phosphate precursor 2 C 2 O 4 and 2.61 g NH 4 H 2 PO 4 , then add 15% sucrose by mass of all raw materials, and continue stirring for 20 min. The mixed gel precursor was obtained after drying in a blast drying oven at 80 °C for 48 h;
[0037] The precursors were ground and placed in a tube furnace, pre-fired at 350 °C for 4 h in an argon atmosphere containing 5% hydrogen, and then ground and mixed uniformly. Then calcined at 550 °C for 12 h in a mixture of...
Embodiment 2
[0040] Example 2: Preparation of Na by sol-gel method combined with high temperature solid phase method 4 Fe 3 (PO 4 ) 2 P 2 O 7 .
[0041] The preparation method of the polyaniline-coated nano-iron phosphate precursor is as in Example 1;
[0042] Add 2.49 g CH to the aforementioned mixed solution 3 COONa, 0.87 g NH 4 H 2 PO 4 And the total mass of these raw materials is about 15% sucrose, and the stirring is continued for 30 minutes. The mixed gel precursor was obtained after drying in a blast drying oven at 80 °C for 48 h;
[0043] In an argon atmosphere containing 5% hydrogen, pre-fire at 400 °C for 4 h, and then grind and mix well. Then calcined in a hydrogen-argon mixture at 550 °C for 12 h to obtain nano-sized Na 4 Fe 3 (PO 4 ) 2 P 2 O 7 .
[0044] The Na prepared above was 4 Fe 3 (PO 4 ) 2 P 2 O 7 The positive electrode powder was fabricated into electrode sheets as described in Example 1, and then assembled into a CR2016 coin cell. The current ...
Embodiment 3
[0045] Example 3: Preparation of Na by spray drying method combined with high temperature solid phase method 3 Fe 2 (PO 4 )P 2 O 7 .
[0046] The preparation method of the iron phosphate precursor is as in Example 1. Add 2.29 gNa to the mixed solution containing the iron phosphate precursor 2 C 2 O 4 , 1.31 g NH 4 H 2 PO 4 And the sucrose of about 15% of the total mass of these raw materials is continuously stirred for 30 minutes, and then spray-dried to obtain a precursor.
[0047] The obtained precursor was pre-calcined at 400 °C for 4 h in a hydrogen-argon mixture (containing 5% hydrogen), ball-milled for 2 h, and then calcined at 550 °C for 12 h to obtain nano-sized Na 3 Fe 2 (PO 4 )P 2 O 7 positive electrode material.
[0048] The prepared positive electrode material was prepared into an electrode sheet according to the method in Example 1, and the battery was assembled and tested. Voltage range of 1.5-4.2 V and current density of 10 mA g -1 The battery ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com