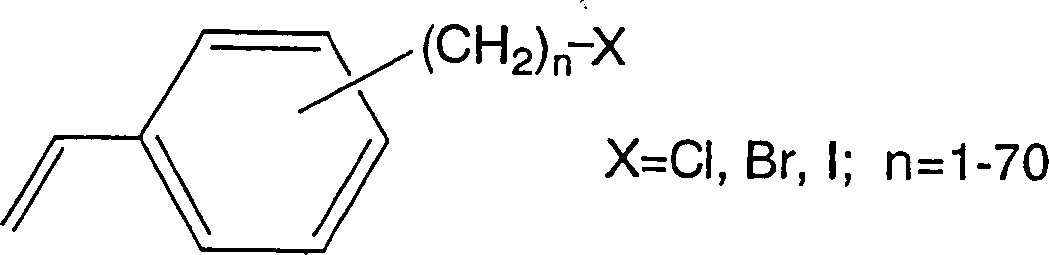Organic polymeric material, process for producing same and heavy-metal ion remover comprising same
一种有机高分子、重金属离子的技术,应用在阳离子交换材料、螯合物的离子交换、离子交换等方向,能够解决残渣后处理、回收和再利用困难、两性物质再溶解、二次污染等问题,达到优异除去性能的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
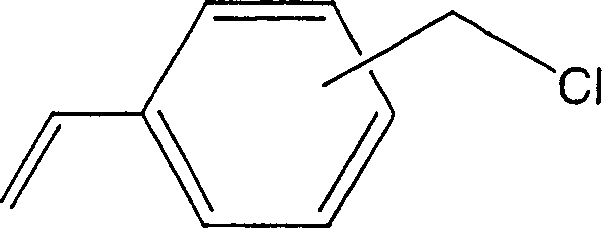
[0035] As an organic polymer base material, a surface density of 50g / m made of polyethylene fibers with a fiber diameter of 10-16μm is used 2 of non-woven fabrics. Under dry ice cooling, 40 g of this nonwoven fabric substrate was irradiated with 160 kGy of γ-rays. The irradiated substrate was impregnated with chloromethylstyrene (50% meta-position, 50% para-position, manufactured by Seimi Chemical Co., Ltd., trade name: CMS-AM) from which the polymerization inhibitor had been removed in advance, and reacted at 50° C. for 5 hours, 86 g of chloromethylstyrene grafted non-woven fabrics with a graft rate of 115% were obtained.
[0036] 19.6 g of this grafted nonwoven fabric was immersed in an isopropanol solution (450 ml) of 52.5 g of diethanolamine, and reacted at 70° C. for 48 hours. After the reacted non-woven fabric substrate was washed successively with pure water, 0.1N aqueous sodium hydroxide solution and methanol, the solvent was removed, and dried under reduced pressure...
Embodiment 2
[0038] 20.7 g of chloromethylstyrene grafted nonwoven fabrics obtained in the same manner as in Example 1 were immersed in acetone solution (400 ml) of 36 g of sodium iodide, and reacted at 50° C. for 48 hours. After the reacted nonwoven fabric substrate was washed with pure water and acetone in this order, the solvent was removed. Subsequently, this nonwoven fabric was immersed in a dimethylformamide solution (360 ml) of 77.8 g of diethyl iminodiacetate, and heated at 80° C. for 48 hours to make it react. The reacted nonwoven fabric was washed with methanol. Furthermore, the non-woven fabric was immersed in a 1N sodium hydroxide-ethanol mixed solution (200ml+200ml), reacted at 80°C for 48 hours, and the non-woven fabric after the reaction was repeatedly washed with pure water, and the water was removed. , dried under reduced pressure at 50° C. to obtain 28.5 g of the organic polymer material of the present invention, which is called heavy metal ion removing agent 2.
Embodiment 3
[0042] Example 3: Evaluation Test of Copper Ion Removal Performance of Heavy Metal Ion Remover in Water (Batch Type)
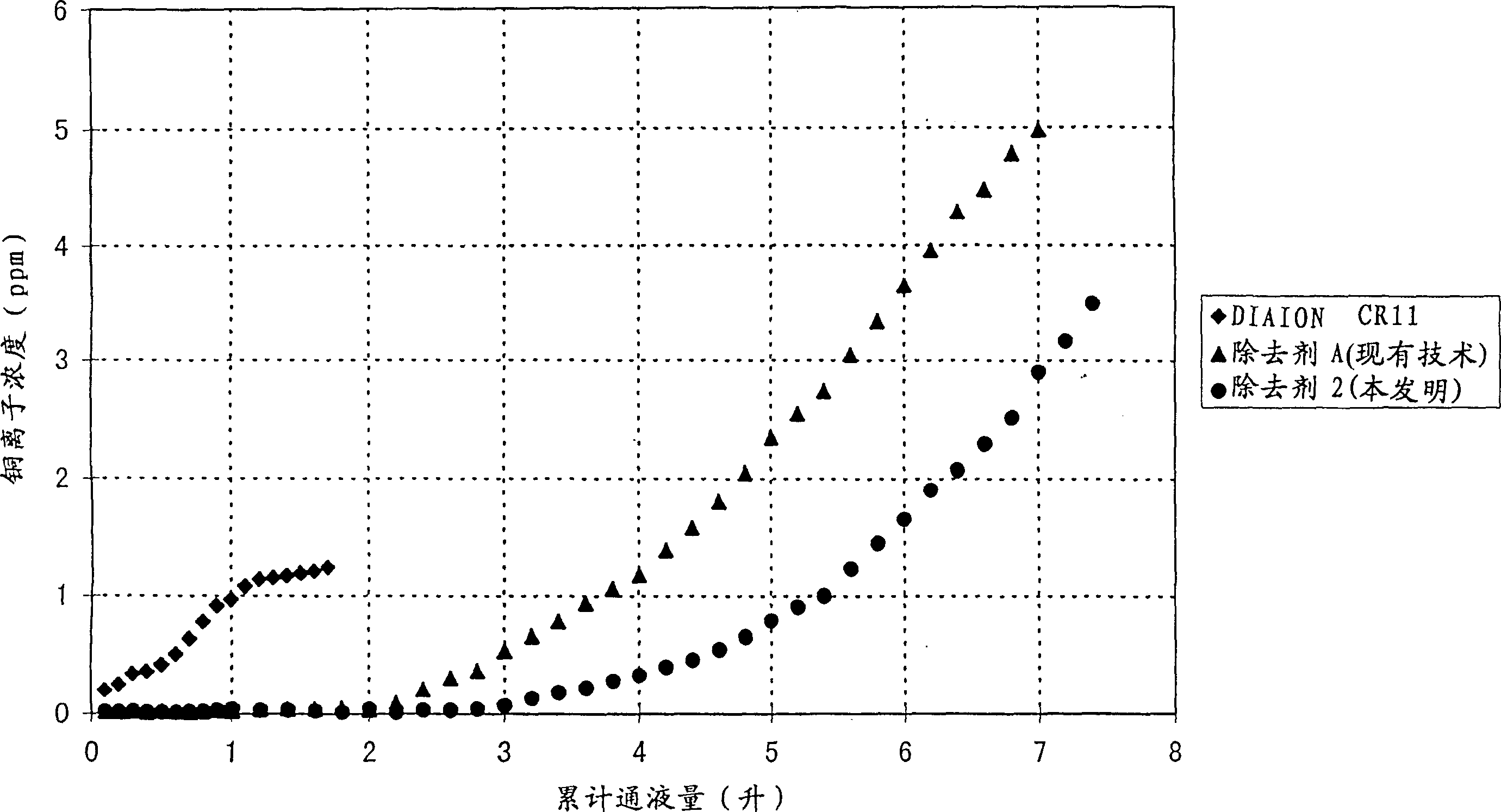
[0043] Copper sulfate was dissolved in pure water to prepare a 100 ppm copper ion aqueous solution (pH=5.15). In an Erlenmeyer flask with a sealing plug, the 9 cm 2 The sample was dipped in 100 ml of this aqueous solution, and stirred well at 25°C. The concentration of copper ions in the aqueous solution after 5 minutes, 15 minutes, 30 minutes, 60 minutes, and 120 minutes after immersion in the removing agent was measured. The results are shown in Table 1.
[0044] Remover 2
[0045] As can be seen from Table 1, the heavy metal ion remover made of the organic polymer material of the present invention has a significantly better removal performance of copper ions than the remover obtained by grafting glycidyl methacrylate in the past.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com