Direct methanol fuel cell stereo electrod and making method thereof
A methanol fuel cell and three-dimensional electrode technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of increased electrode and membrane contact resistance, fuel loss, and affecting the performance of cathode catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
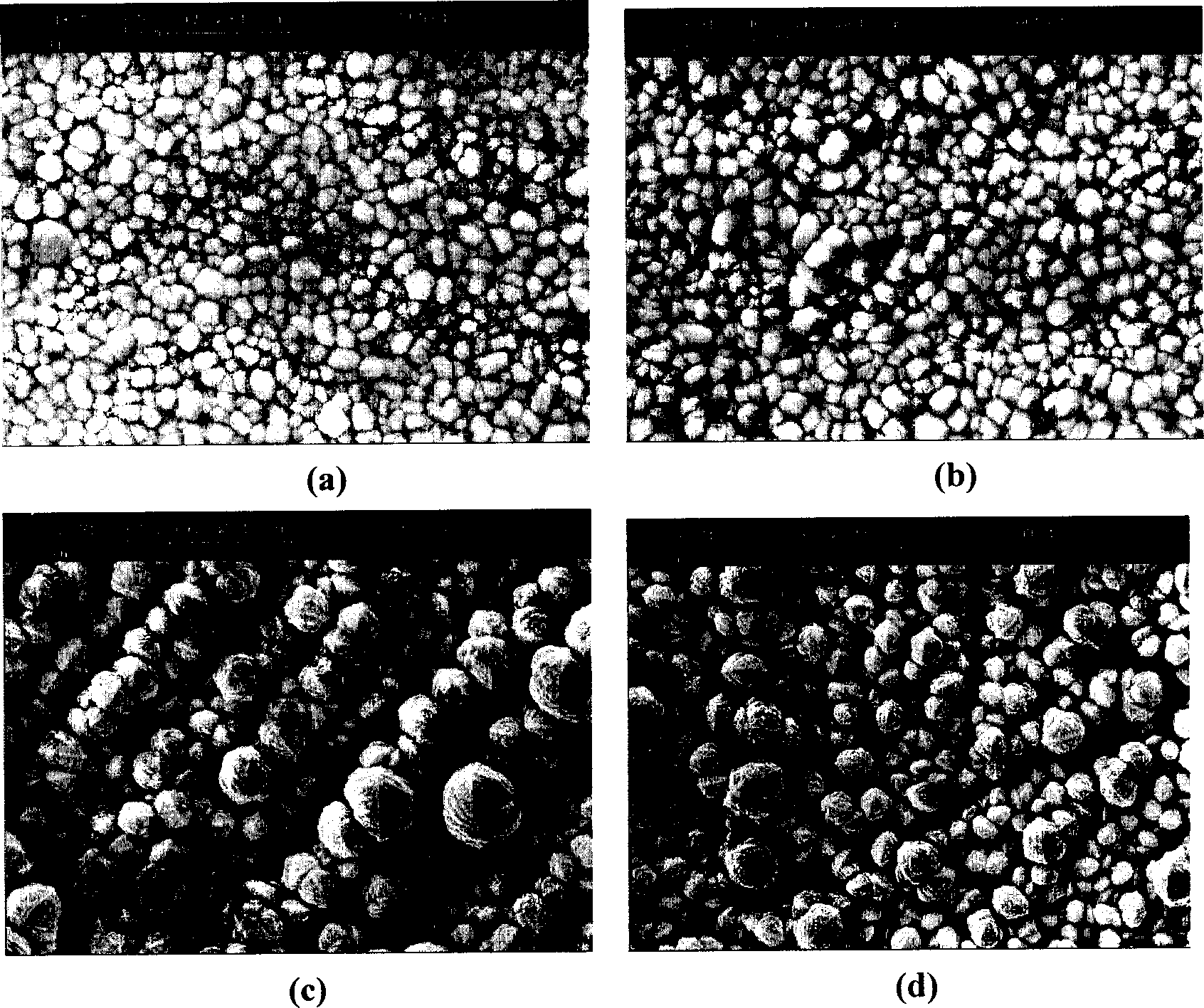
[0016] Example 1: Preparation of Pt / WO by constant current reduction method 3 Stereo electrode
[0017] Dissolve tungsten powder with hydrogen peroxide solution, and excess hydrogen peroxide is inserted into platinum black electrode to decompose. Prepare an aqueous solution containing 50 mmol tungsten, 4-8 mmol platinum, and 30% isopropanol, and then reduce it to Pt / WO under constant current 3 Stereo electrode for direct methanol fuel cell. figure 1 is in the above solution (4mmol dm -3 The scanning electron micrograph of the catalyst obtained by Pt) electrochemical deposition, wherein figure 1 a is at -0.25mA cm -2 The current density was reduced for ten minutes. It can be seen that the surface of the sediment layer is relatively dense, and the real specific surface area is not much different from its geometric area. When the current density reaches -2.5mA cm -2 Time( figure 1 b), the reduction speed is greatly accelerated, and the sediment is too late to be reduced r...
Embodiment 2
[0018] Embodiment two: the preparation of Pt / Pb / PbxOy three-dimensional electrode
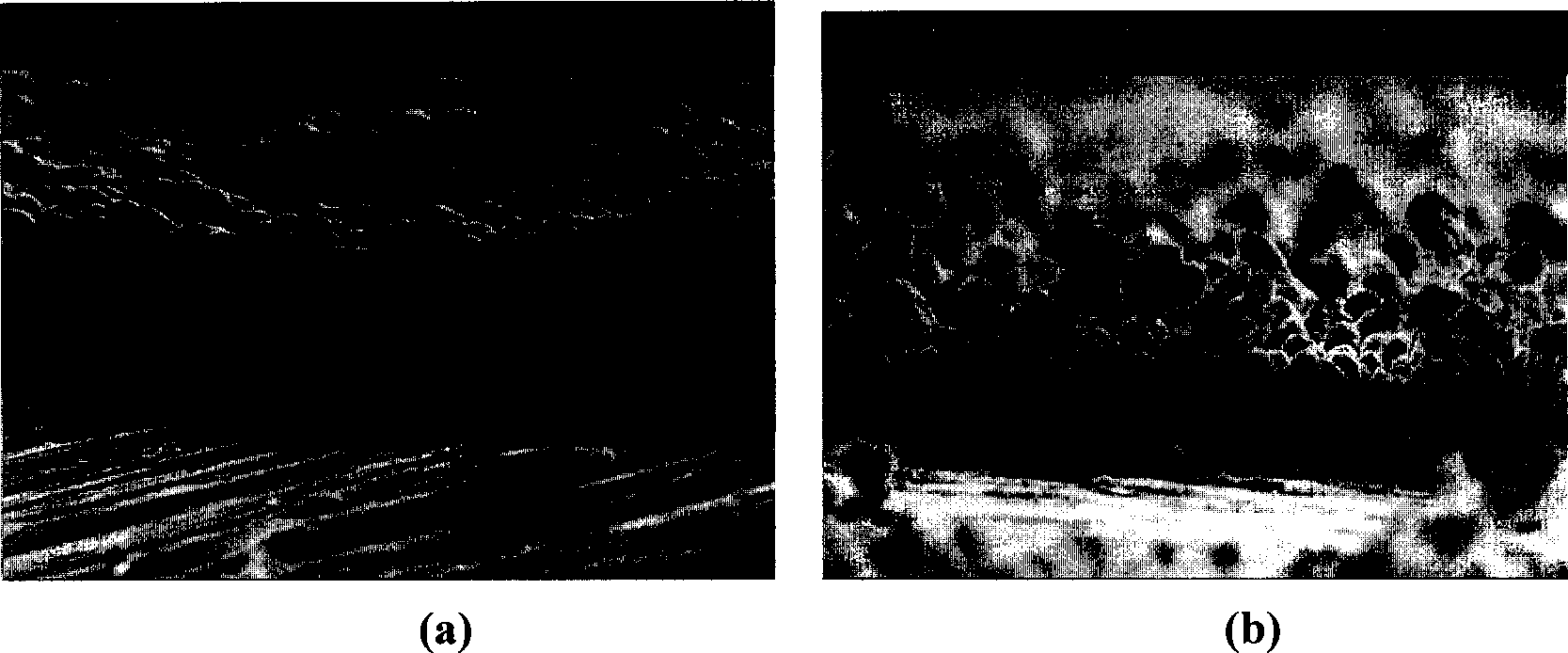
[0019] Under special conditions, lead can be prepared into rod-shaped oxides to form a porous structure with high specific surface area. The application of such a structure in lead-acid batteries will improve the specific energy and charge-discharge performance of the battery. In this example, first prepare the following solution: 0.1mol dm -3 Pb(NO 3 ) 2 +0.2mol dm -3 HClO 4 +0.01mol dm -3 NaF in water containing 30 v / v % methanol and 1 v / v % of 5% Nafion suspension (Du Pont). Then carry out oxidation treatment in an electrolytic cell with platinum as counter electrode and saturated calomel electrode as reference electrode to prepare lead oxide. Electrodes deposited with lead oxide are further modified with noble metals to be catalytically active (step-by-step approach). figure 2 The SEM pictures shown are the electrode surface topography obtained on two different substrate materials and...
Embodiment 3
[0021] Example 3: Potentiostatic construction of PtRu / WO 3 Stereo electrode
[0022] Platinum-ruthenium alloy catalysts are currently recognized as the most suitable catalysts for proton exchange membrane fuel cells and direct methanol fuel cells. The tungsten trioxide-supported platinum-based noble metal catalyst disclosed in the world invention patent (WO92 / 16027) not only has high catalytic activity, but also It has strong anti-carbon monoxide poisoning characteristics. However, the specific surface area of the catalyst prepared by the electrochemical method at low current density is relatively small, and the activity is low. Accordingly, in this example, the deposition conditions were changed to prepare PtRu / WO 3 catalysts, and compared the surface morphology of catalyst layers obtained under different conditions. Figure 4 Co-deposition (one-step method) to prepare PtRu / WO under different reduction potentials 3 The surface morphology of the material. Figure 4 a is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com