Preparation for hydrophobic solid acid catalyst
A technology for solid acid catalysts and hydrophobic solids, which is applied in catalyst activation/preparation, molecular sieve catalysts, chemical instruments and methods, etc. It can solve the problems that the hydrophobicity of solid acid catalysts has not been reported, and achieve excellent catalytic effect and thermal stability. Good, the effect of shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Weigh 0.70g of ferric sulfate in a beaker, add 8ml of deionized water to dissolve; weigh 2.79g of HZSM-5 in a suction filter bottle, the ratio of silicon oxide to aluminum oxide is 60, vacuumize for 2h, and dissolve the sulfuric acid Add the iron solution dropwise, stir at 45°C, impregnate for 24h, and roast at 200°C for 4h, and obtain a 20% solid acid catalyst by impregnating at room temperature; put 0.1mol catechol, 0.2mol tert-butanol and 0.55g catalyst into the reactor, heat , electromagnetic stirring, reacted at 120°C for 2h, cooled, and the reaction solution was centrifuged to separate the catalyst and analyzed by gas chromatography. The conversion rate of catechol was 79.5%, and the selectivity of tert-butyl catechol could reach 87.8%.
Embodiment 2
[0032] Example 2: Weigh 0.97g of ferric chloride in a beaker, add 8ml of deionized water to dissolve; weigh 2.26g of HZSM-5 in a suction filter bottle, the ratio of silicon oxide to aluminum oxide is 60, vacuumize for 2h, and Ferric chloride solution was dripped, stirred at 25°C, impregnated for 36 hours, dried at 60°C, roasted in a muffle furnace at 200°C for 2 hours, and obtained a 30% solid acid catalyst by impregnation at room temperature; Add 0.2mol α-pinene, 0.2mol chloroacetic acid, 0.25mol water and 0.89g catalyst into a 250ml three-necked flask with a thermometer, react at 60°C for 8 hours, after cooling, filter out the catalyst, neutralize it with saturated sodium carbonate solution, and let it stand Separate the layers, wash the organic layer with water until neutral, and dry it with anhydrous magnesium sulfate to obtain a clear liquid. According to gas chromatography analysis, the conversion rate of the product α-pinene is 54.6%, and the selectivity of α-terpineol i...
Embodiment 3
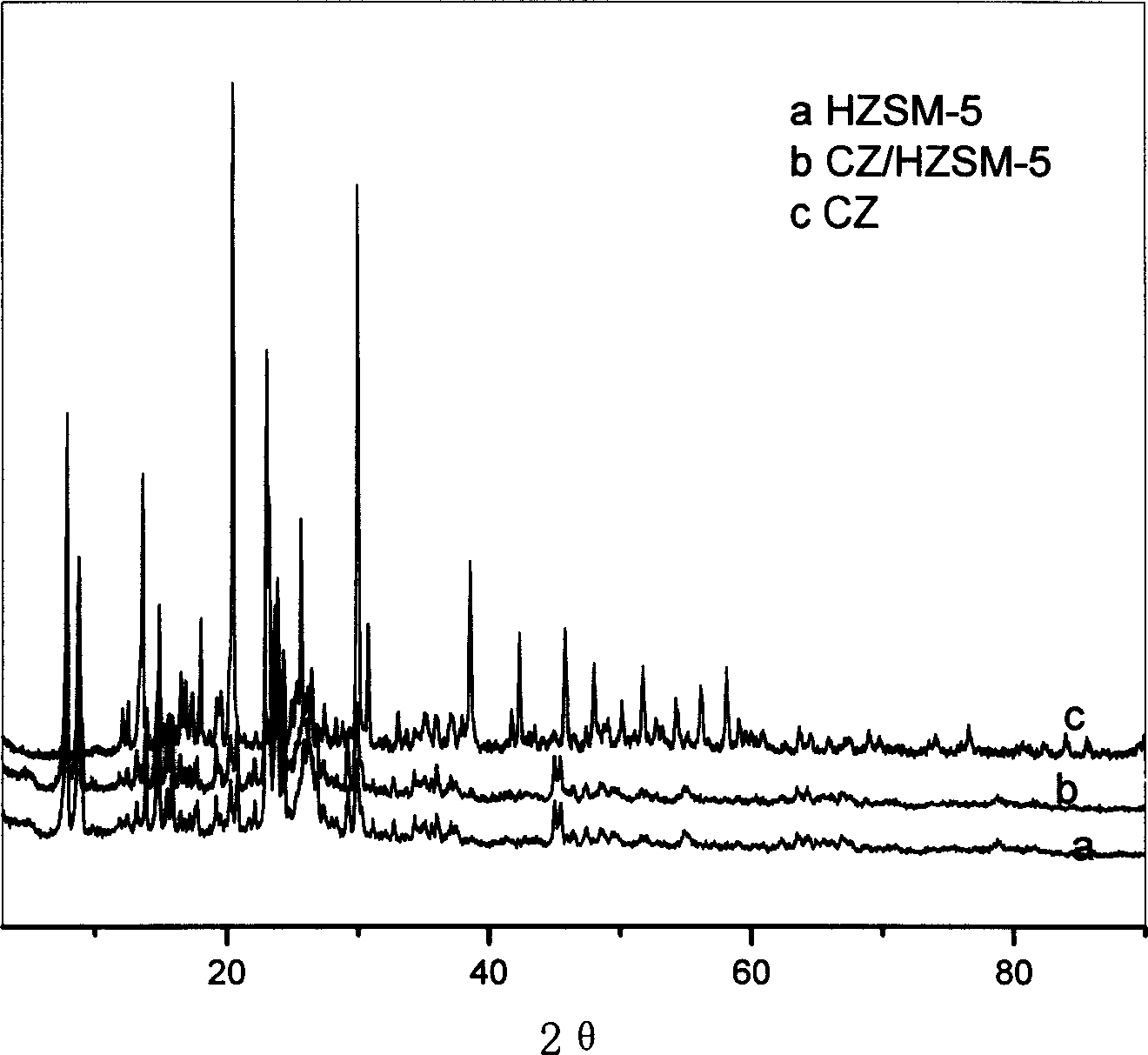
[0033] Example 3: Weigh 0.47g of zirconium sulfate in a beaker, add 10ml of deionized water to dissolve; weigh 1.87g of HZSM-5 in a suction filter bottle, the ratio of silicon oxide to aluminum oxide is 120, vacuumize the zirconium sulfate The solution was dripped, stirred at 30°C, impregnated for 24h, roasted at 110°C, and a 20% solid acid catalyst was obtained by impregnating at room temperature. It appears in -5, indicating that the active component zirconium sulfate tetrahydrate is highly dispersed into the pores of HZSM-5, see the description of the drawings in the specification figure 1 0.118mol n-octanol, 0.135mol acrylic acid, 18ml toluene, 0.2g p-methoxyphenol and 2.0g self-made catalyst are added in the three-necked flask of 100ml, a thermometer, a water separator, a reflux condenser and an air duct are installed, and the Enter air, control the air flow rate to 20ml / min, start magnetic stirring, heat up to 125°C, and react until the water output no longer increases, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com