GP thymosin alpha 1 and preparation method
A technology of thymosin and Escherichia coli, which is applied in the field of medical bioengineering, can solve the problems of environmental pollution and high cost of chemical synthesis, and achieve the effect of simple preparation method, low cost and good biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
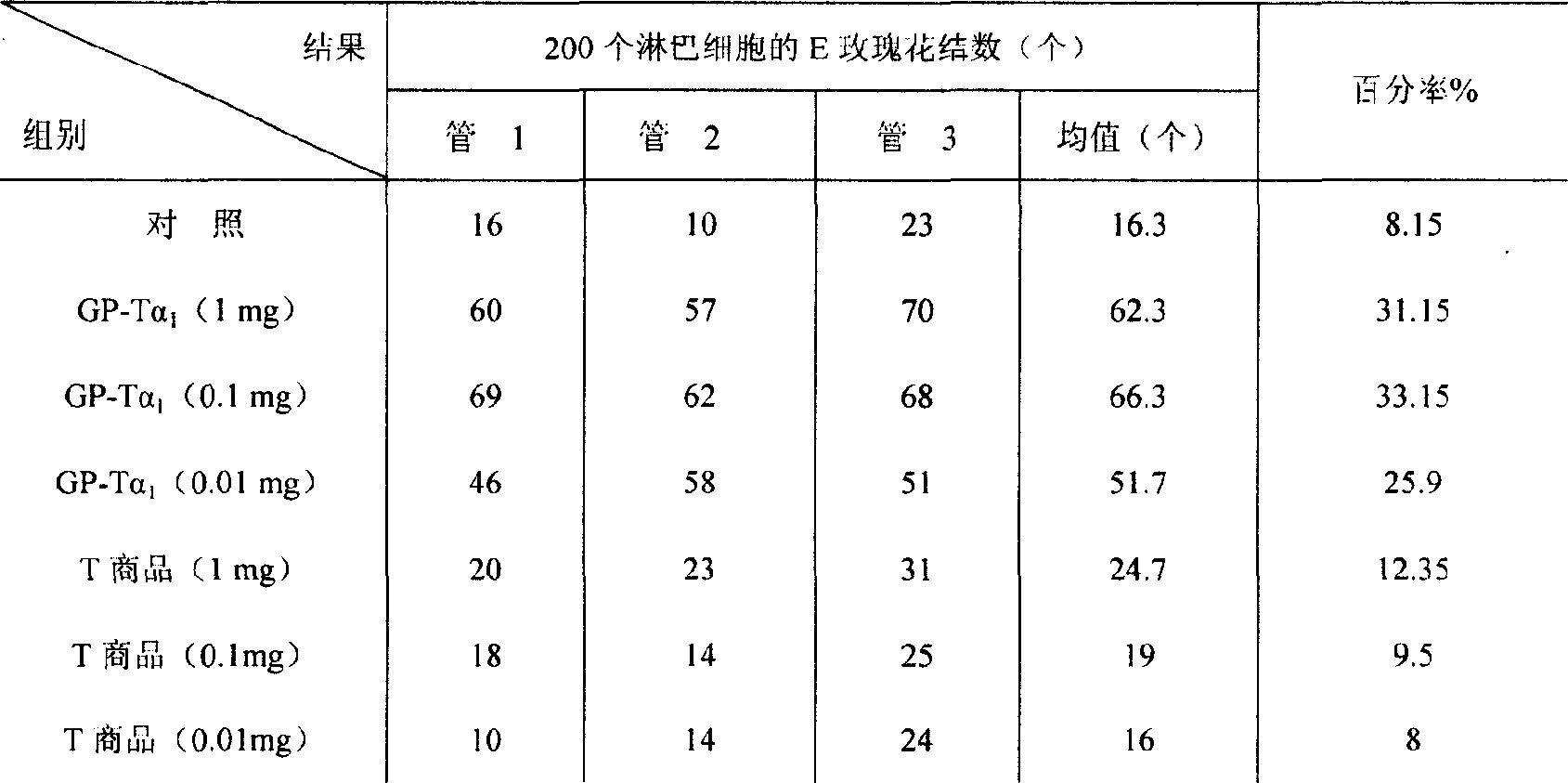
Examples
Embodiment 1
[0013] Example 1: Construction of fusion expression plasmid pTRX-GP-Tα1
[0014] 1. Reagents and materials
[0015] The vector plasmid pTRX was constructed by Dr. Peng Lisheng from the School of Life Sciences, Sun Yat-sen University, and has applied for a national invention patent with application number 00124832; Escherichia coli BL21 (DE3) was purchased from Stratagene, and the thymosin α1 gene was synthesized by Shanghai Boya Biotechnology Company ; Restriction endonuclease and T4DNA ligase were purchased from New England Company, PCR primers were synthesized by Shanghai Boya Biotechnology Company, and gel recovery kit was purchased from Omega Biotechnology Company.
[0016] 2. Method
[0017] (1) Oligonucleotide primer:
[0018] Upstream primer: 5’-GG GGTACC TCTGATGCTGCGGTCGAT-3’
[0019] Downstream primer: 5’-GTCAT GCGGCCGC GTTCTCGGCTTCTTCCACAA-3’
[0020] The upstream primer contains Kpn I restriction site (single underline), PreScission Protease (3C) recognition site (do...
Embodiment 2
[0026] Example 2: Fermentation of Escherichia coli engineered strain DE3 / pTRX-GP-Tα1
[0027] 1. Engineering bacteria and plasmids: engineering bacteria BL21(DE3) / pTRX-GP-Tα1;
[0028] 2. Medium:
[0029] LB medium (g / L) for the activation of first-level seed bacteria: peptone 10g, yeast powder 5g, NaCl 10g;
[0030] 2YT medium for activation of secondary seed bacteria (g / L): peptone 16g, yeast powder 10g, NaCl 5g;
[0031] Fermentation tank fermentation medium (g / L): peptone 12g, yeast powder 12g, NaCl 5g, KH 2 PO 4 4g, MgSO 4 ·7H 2 O1g, K 2 HPO 4 5g, 2g glucose; mix the above medium during fermentation;
[0032] Feeding carbon source (400ml): 40g glucose, MgSO 4 ·7H 2 O 3g;
[0033] Feed nitrogen source (g / L): 37g peptone, 37g yeast powder;
[0034]3. Activation of seed bacteria: streak the engineered strains preserved in -70°C and 20% glycerol, culture at 37°C for about 16 hours, pick a single clone, and inoculate it in 200ml LB medium (containing 100μg / ml ampicillin) In an Erl...
Embodiment 3
[0042] Example 3: Preparation and purification of recombinant GP-Tα1 polypeptide:
[0043] 1. Centrifuge the fermentation broth to collect the bacteria, and resuspend the bacteria with 50mM Tris-HCl (pH8.0), 0.5M NaCl solution at the ratio of 10ml solution / g wet bacteria. Branson 450 ultrasonic cell crusher was used to break the bacteria, the probe model is 1 / 2", ultrasonic 15-25min (ultrasonic 8sec, intermittent 4sec) at 70% power in ice bath. Centrifuge at 4℃, 10,000rpm for 30min (BECKMAN AVANTI TM J-25), collect the supernatant.
[0044] 2. Supernatant Ni 2+ -Chelating Sepharose affinity chromatography column, discarding the passing peak; eluting with Tris-HCl (pH 7.0), 0.5M NaCl, 20 mM imidazole solution, until the plateau; using Tris-HCl (pH 7.0), 0.5M NaCl, 150mM imidazole solution was eluted, and the elution peaks were collected; the Sephadex G-25 column was replaced with 20mM Tris-HCl (pH 8.0), 20mM NaCl buffer, and then on the Q Sepharose Fast Flow column, with 50mM Tris-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com