Polymer, chemical range enlargement erosion resistant component and forming method
A technology of polymer and mixture, applied in the field of polymer, chemically amplified resist composition and formation, can solve the problems of uneven circuit, line obstacle, long dissolution time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0367] In a 500ml eggplant-shaped flask equipped with a circulating cooling tube, add 2,5 dimethyl 2-(1-adamant compound)-1,3-dioxolan-4-one 25.0g (0.1 mol); add bromine 19.6 g (0.11 mol) of succinimide; 240 g of heptane was added, and the reaction temperature was raised to 60° C. while stirring to form a paste.
[0368] After stirring at 60° C. for about 2 hours, the color of the reaction solution turned brown, and after continuing to stir for 2 hours, the color of the reaction solution turned transparent. After cooling the reaction solution in an ice bath, filter to remove succinic acid imide, distill off the solvent, and purify the obtained transparent liquid with a column to obtain 5-bromo-2,5-dimethyl 2-(1-adamantine compound )-1,3-dioxolan-4-one 28.6g (Formula 18), (87% recovery).
[0369]
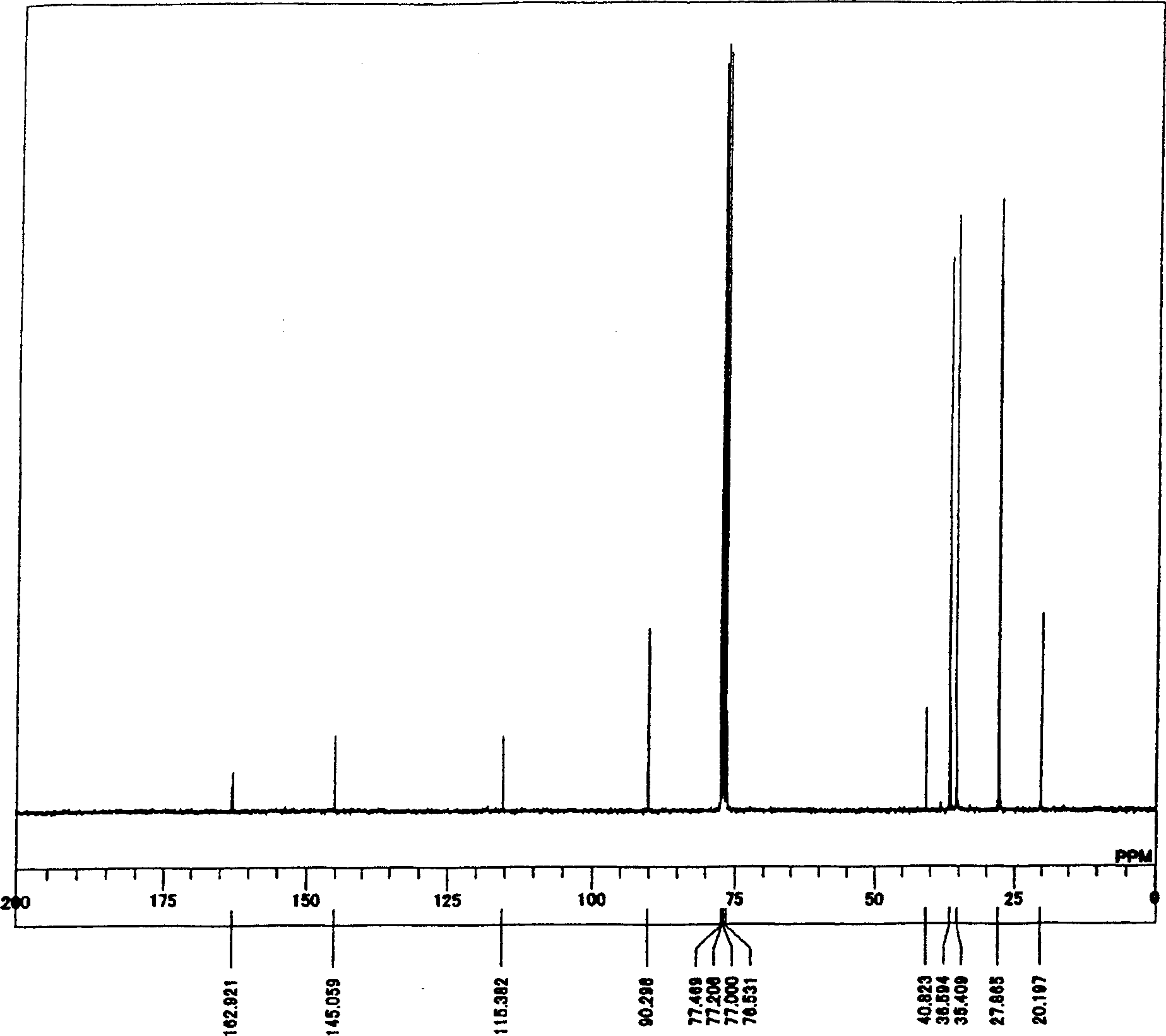
[0370] Elemental analysis: C54.56%, H6.64%, N0%, O14.73%.
[0371] Theoretical values: C54.72%, H6.43%, N0%, O14.58%.
[0372] Br content (flask combustion ion chromatography):...
Embodiment 2
[0380] In a 500ml eggplant-shaped flask equipped with a reflux cooling tube, add 22.2g (0.1mol ); add 21.4 g (0.12 mol) of bromosuccinimide; add 200 g of cyclohexane, and stir to form a paste while raising the reaction temperature to 60°C.
[0381] After stirring at 60° C. for about 2 hours, the color of the reaction solution turned brown, and continued stirring for another 2 hours, and the color of the reaction solution turned transparent. After cooling the reaction liquid in an ice bath, succinimide was removed by filtration, and the solvent was distilled off. The precipitated crystals were recrystallized with hexane to obtain 25.6 g of spiro[adamantane-2,2'-(5'-methanol-1',3'-dioxolan-4-one)] (Formula 20) , (85% recovery).
[0382]
[0383] Elemental analysis: C51.70%, H5.82%, N0%, O16.19%.
[0384] Theoretical values: C51.84%, H5.69%, N0%, O16.94%.
[0385] Br content (flask combustion ion chromatography): 25.4% (theoretical value 26.53%).
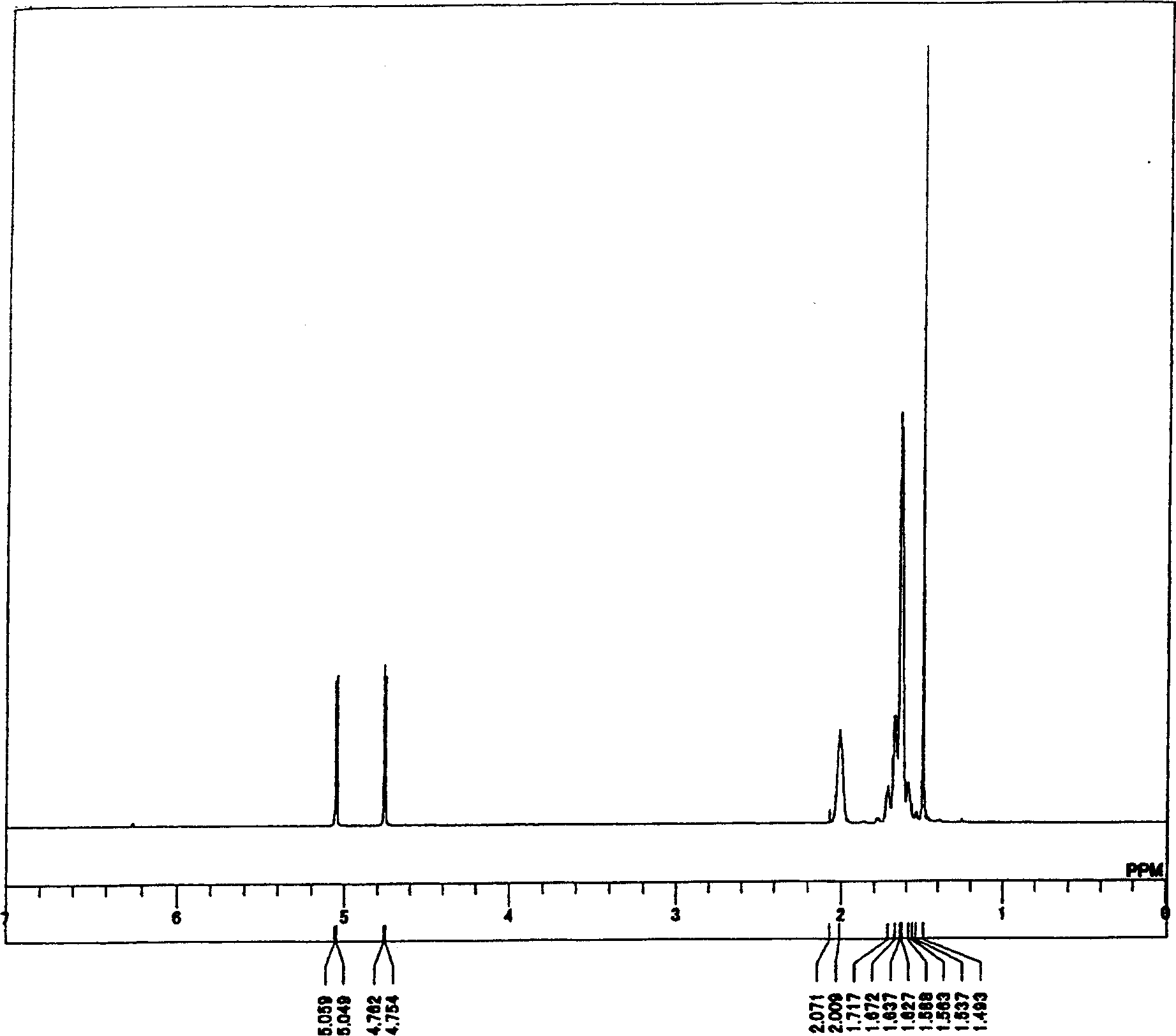
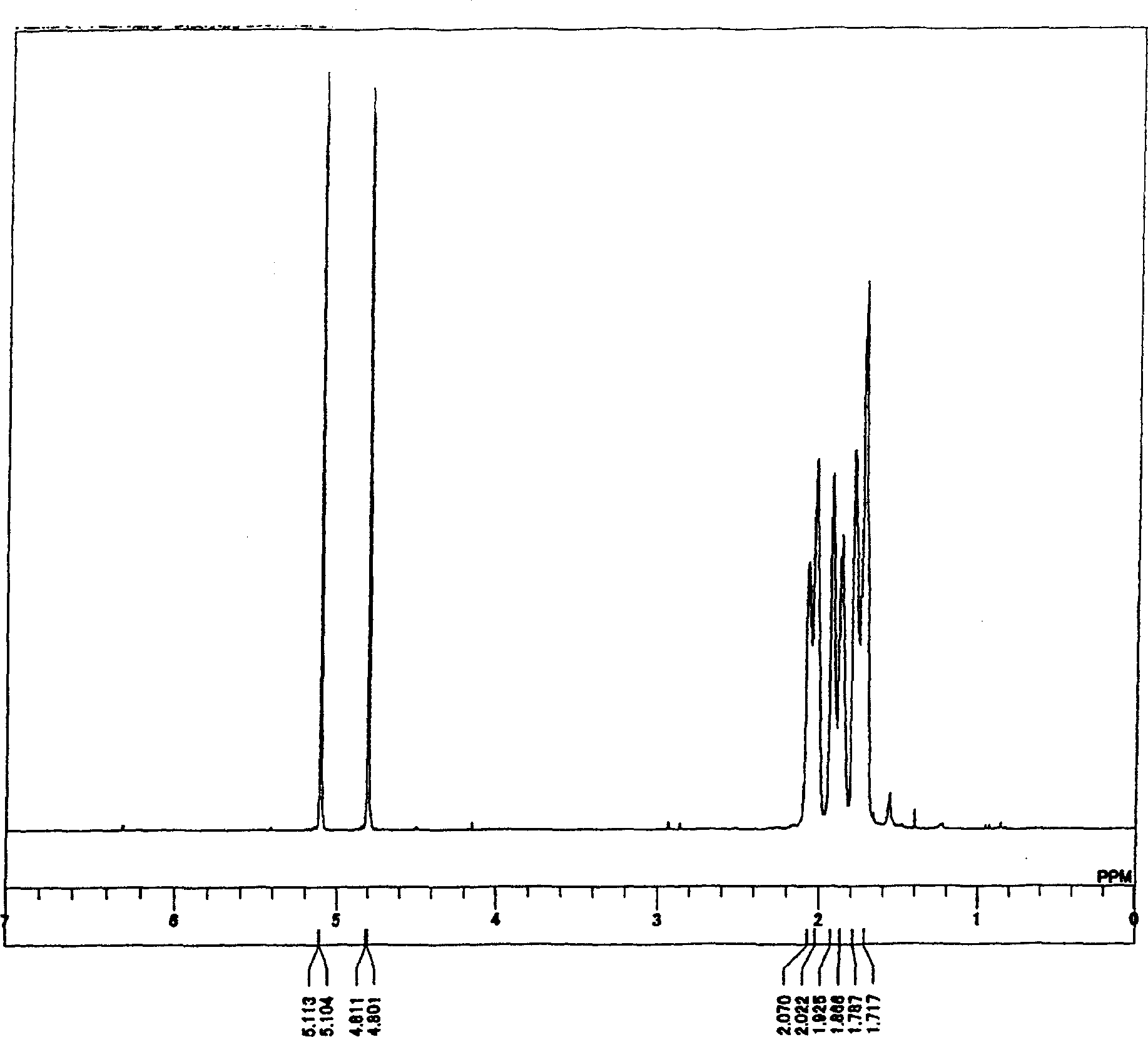
[0386] H-NMR (270MHz, -...
Embodiment 3
[0394] In a 500ml eggplant-shaped flask equipped with a reflux cooling tube, add spiro[norbornane-2,2'-(5'-methanol-1',3'-dioxolane-4'-one)] 18.2 g (0.1 mol); add 16.9 g (0.11 mol) of N-bromosuccinimide; add 280 g of cyclohexane, and stir to form a paste while raising the reaction temperature to 60°C.
[0395] After stirring at 60° C. for about 2 hours, the color of the reaction solution turned brown, and continued stirring for another 2 hours, and the color of the reaction solution turned transparent. After the reaction solution was cooled in an ice bath, the analysis result contained 25.1 g of spiro[norbornane-2,2'-(5'-methanol-1',3'-dioxolane-4'-one)], (Formula 22), (96% recovery).
[0396]
[0397] After transferring the obtained liquid into a 100 ml eggplant-shaped flask, 70.0 g (1.0 mol) of N·N-dimethylamide was added, followed by vigorous stirring at room temperature for 2 hours. The succinimide produced in the previous step was dissolved in N-dimethylamide while s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com