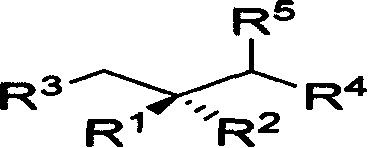
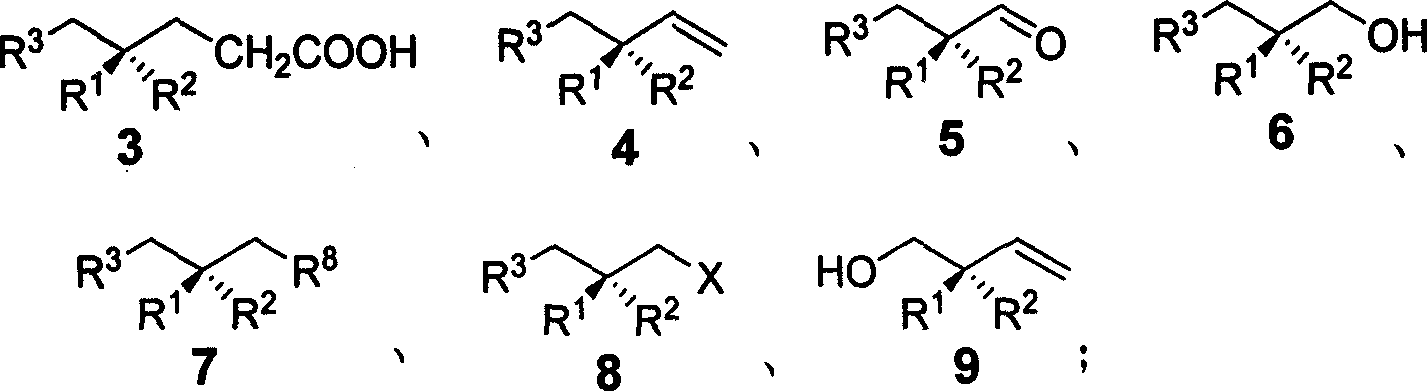
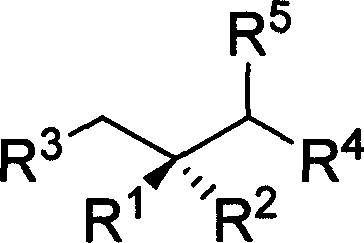
Method for synthesizing chiral methyl 1,3 functional group synthon
A synthesis method and functional group technology, applied in the field of synthesis of a class of chiral methyl 1,3-functional group synthons, can solve the problems of troublesome operation, expensive reagents, and low yield of a single isomer, and reduce pollution , the effect of the simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The synthesis of embodiment 1 compound 3a
[0031]
[0032] Dissolve 23g (0.2mol) of reactant 1a in 400ml of toluene, add 24g (0.6mol) of NaOH, heat up to 80-90°C, react for about 1h, then add 37.6g (0.22mol) of BnBr, heat to reflux, azeotropically remove water , about 20h after the reaction is completed, cool to room temperature, add water to dissolve all the solids, and then use Et 2 O extraction, recovery of raw materials and BnBr, the water phase was acidified with hydrochloric acid and CH 2 Cl 2 Extraction, the extract was washed with saturated brine, anhydrous Na 2 SO 4 dry. After filtering and spin-drying, 30.4 g of light brown liquid compound 3a was obtained, with a yield of 68%.
[0033] Compound 3a: C 13 h 18 o 3 ;FW222;
[0034] 1 H-NMR (CDCl 3 , 300MHz) δ: 7.26-7.35(m, 5H), 4.50(s, 2H), 3.31(d, 2H, J=5.7Hz), 2.36-2.43(m, 2H), 1.78-1.88(m, 2H) , 1.47-1.55(m, 1H), 0.95(d, 3H, J=5.7Hz).
Embodiment 2
[0035] The synthesis of embodiment 2 compound 3c
[0036]
[0037] Dissolve 1.6g (6.2mmol) of compound 2c in 10mL of ethanol, add 1.6g of NaOH in 20mL of aqueous solution, and heat to reflux for 1h. After the reaction is complete, add hydrochloric acid to adjust the pH to 2-3, extract with ethyl acetate, and wash the organic phase with saturated NaCl solution. Anhydrous Na 2 SO 4 dry. After filtration, the solvent was spun off to obtain 1.4 g of compound 3c, with a yield of 92%.
[0038] Compound 3c: C 12 h 26 o 3 Si; FW246;
[0039] 1 H-NMR (CDCl 3 , 300MHz) δ: 4.44(d, 2H, J=6.0Hz), 2.36-2.43(m, 2H), 1.74-1.79(m, 1H), 1.61-1.68(m, 1H), 1.44-1.49(m, 1H), 0.89-0.91(d, 3H, J=6.6Hz), 0.89(s, 9H), 0.04(s, 6H);
[0040] MS (EI, m / z): 247 (M + +1).
Embodiment 3
[0041] The synthesis of embodiment 3 compound 4a
[0042]
[0043] 9.0g (20mmol) Pb (OAc) 4 , 0.4g (2mmol) Cu(OAc) 2 ·H 2 O, 0.8g (10mmol) of pyridine, 2.22g (10mmol) of compound 3a were dissolved in 70ml of toluene, heated to reflux, reacted for about 4h, cooled to room temperature, filtered with diatomaceous earth, and the filter residue was washed with a large amount of ethyl acetate, and the washing liquid was mixed with The filtrates were combined, washed successively with 1N HCl, saturated brine, anhydrous Na 2 SO 4 dry. After filtration and spin to remove the solvent, column chromatography separated to obtain 0.788 g of light yellow liquid compound 4a with a yield of 44.8%, and recovered 1.07 g (46.7%) of compound 3a.
[0044] Compound 4a: C 12 h 16 O;FW176;
[0045] [α] D 23 =5.8° (c=2.10, CHCl 3 );
[0046] 1 H-NMR (400MHz, CDCl 3 )δ: 7.35 (m, 4H), 7.26-7.30 (m, 1H), 5.78-5.87 (ddd, 1H, J = 17.3, 10.4, 7.0Hz), 5.06-5.11 (d, 1H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com