Fuel cell membrane electrode assemblies with improved power outputs and poison resistance
a fuel cell membrane electrode and power output technology, applied in the direction of cell components, electrochemical generators, vacuum evaporation coating, etc., can solve the problems of power output loss, initial cost, and longening the operational li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
second embodiment
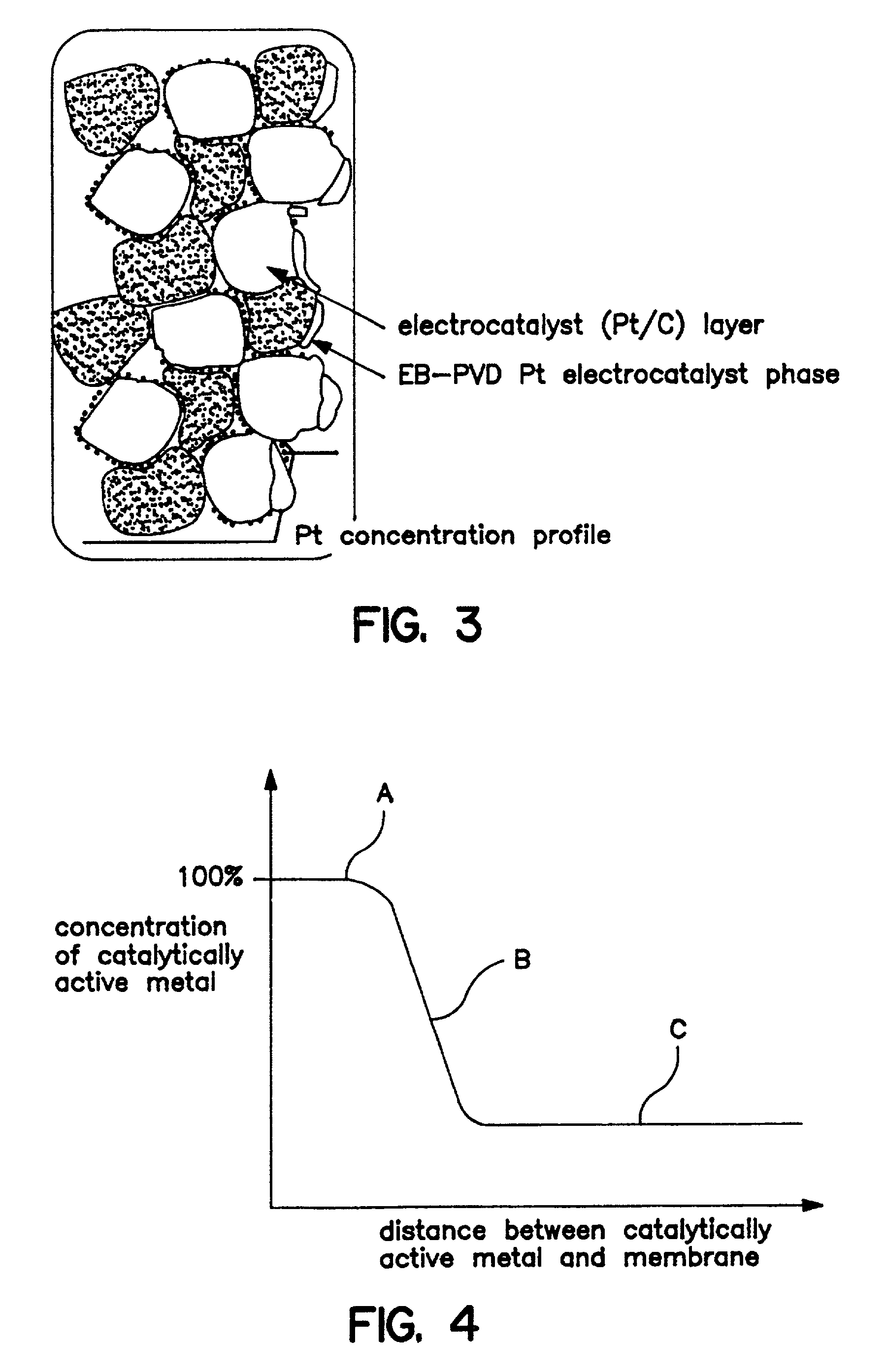
[0164] In alternative embodiments, however, which are now described, the electrode of the membrane-electrode combination is substantially free of first catalytically active metal, and the zone of second catalytically active metal includes at least two different catalytically active metals which helps improve resistance to poisoning. Hence, the primary, and preferably essentially all, catalytic activity is from the zone. These alternative embodiments, wherein the electrode is substantially free of first catalytically active metal, are called collectively for purposes herein "THE SECOND EMBODIMENT" and are described further hereinbelow.
[0165] In the second embodiment, preferably, the amount of first catalytically active metal is minimized so that the electrode is substantially free of first catalytically active metal, and preferably, totally free. More specifically, catalytic loading for the first catalytically active metal can be less than about 0.1 mg / cm.sup.2, and preferably less t...
first embodiment
[0170] Deposition methods are noted above in the description of the first embodiment and include sputtering and thermal evaporation. In a multi-metallic physical vapor deposition, such as electron beam physical vapor deposition, separate metal sources of about 99.9 wt. % platinum and about 99.9 wt. % ruthenium are evaporated. Preferably, for a bimetallic mixture of these metals, the atomic ratio of each metal is about 1:1. Simultaneous or sequential physical vapor deposition methods can be used. In simultaneous deposition, two different sources are used and simultaneously evaporated together, and the vapor composition is controlled by each source's relative evaporation rate. In sequential deposition, each metal is separately evaporated from its source in a toggle-like manner and deposited onto the chosen substrate in alternate thin layers. Alloying of the metals can be encouraged when the layers are thin enough. In addition, ion bombardment treatment such as, for example, ion beam a...
example 1
[0191] Example 1 illustrates the indirect method wherein the zone of second catalytically active metal is first deposited onto a substrate before transfer from the substrate to the membrane or electrode.
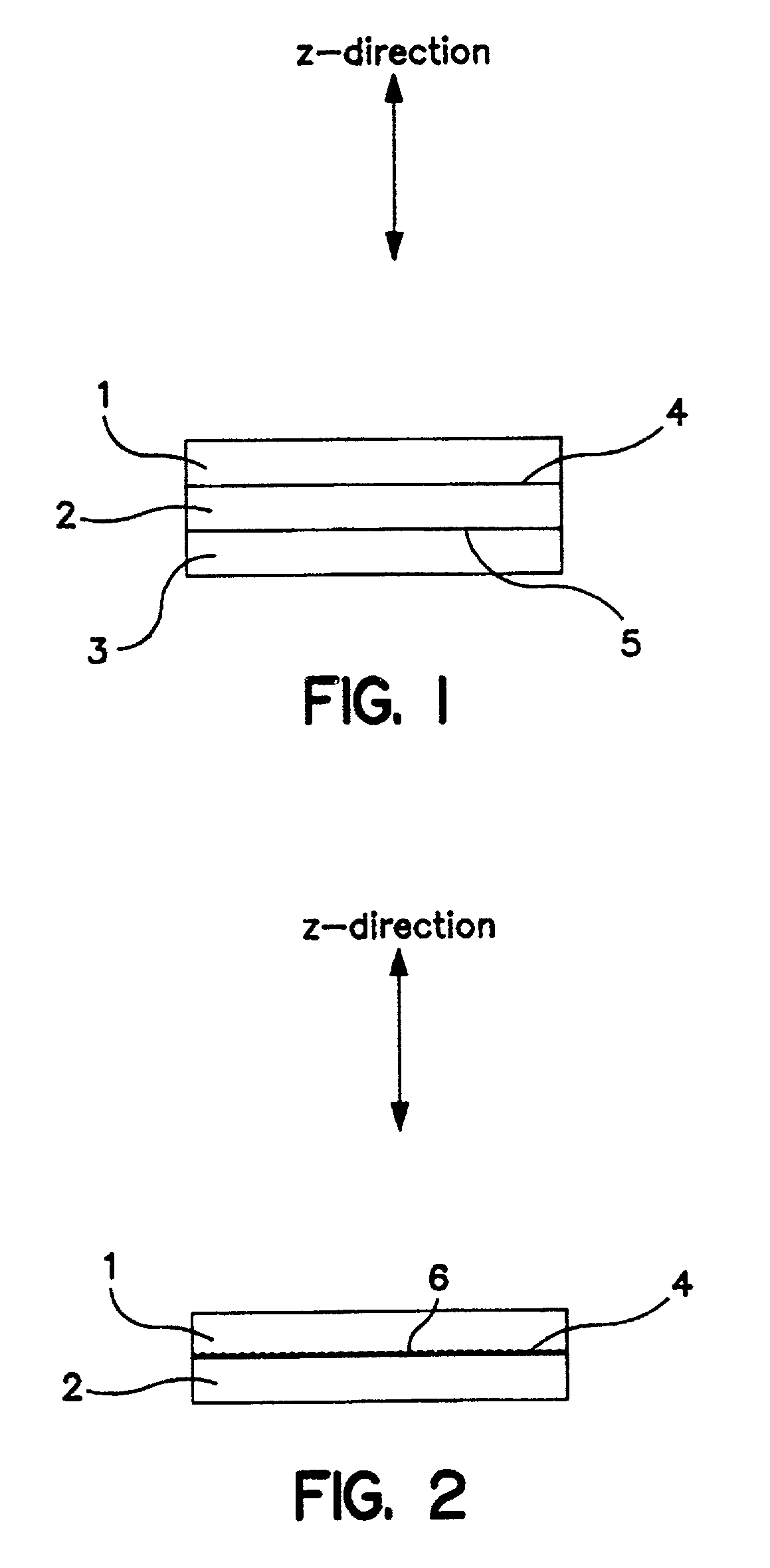
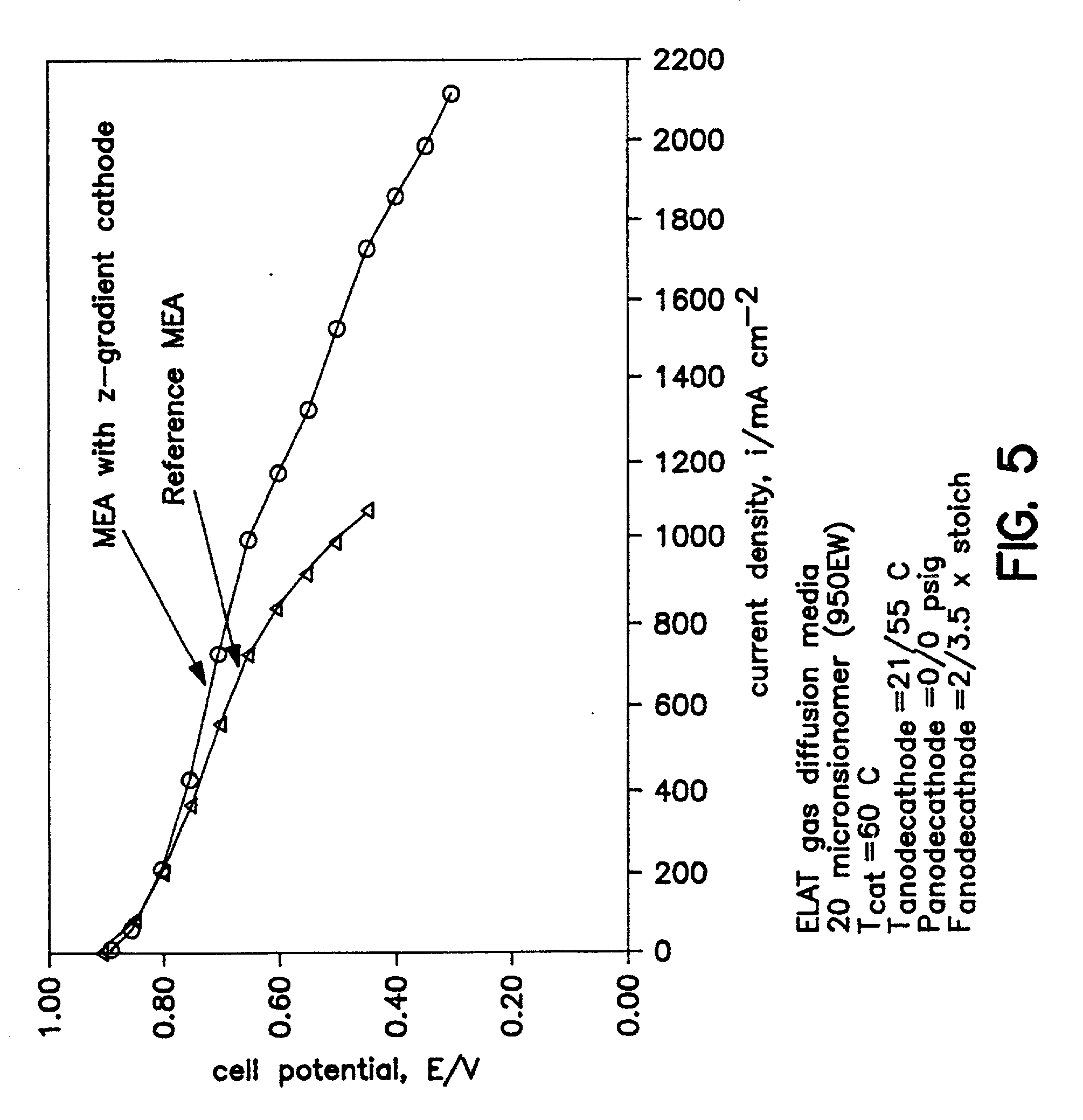
[0192] A 50 .ANG. platinum coating zone (0.01 mg / cm.sup.2) was deposited at 1 .ANG. / sec onto a skived PTFE substrate backing by EB-PVD. The catalyst zone was then transferred onto the membrane by the decal method leaving the 50 .ANG. catalyst zone bonded to one side of the membrane and positioned centrally. The area of the membrane demarcated by the transferred catalyst is the active area. A catalyzed electrode (0.3 mg Pt / cm.sup.2) was attached to each side of the catalyzed membrane also using the decal method, so as to overlay the active area. Therefore, one side of the MEA had a z-gradient zone of platinum at the membrane / electrode interface.
[0193] The prepared MEAs with 25 cm.sup.2 active areas were each loaded between gaskets in a 25 cm.sup.2 active area fuel cell test fixture or...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com