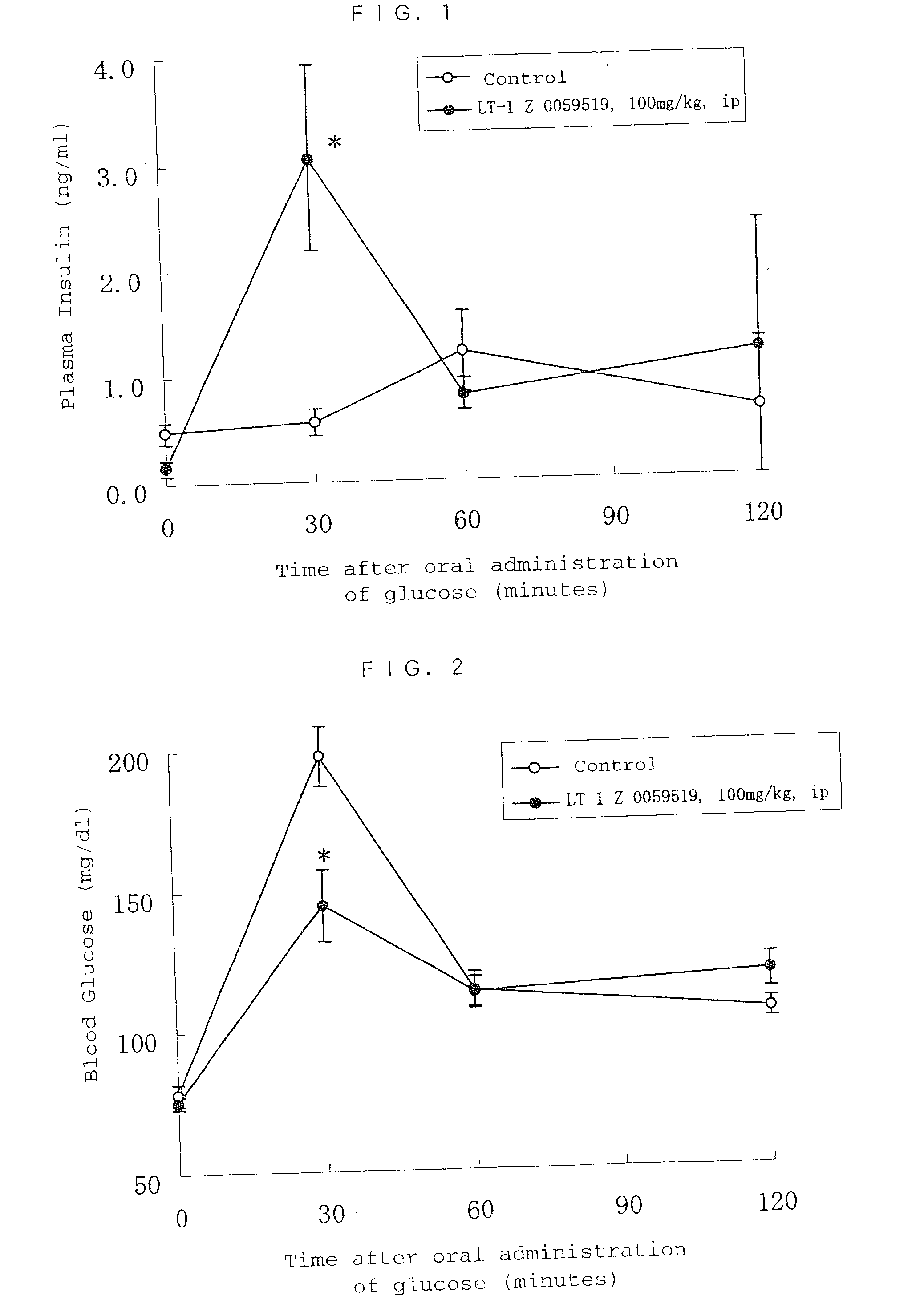
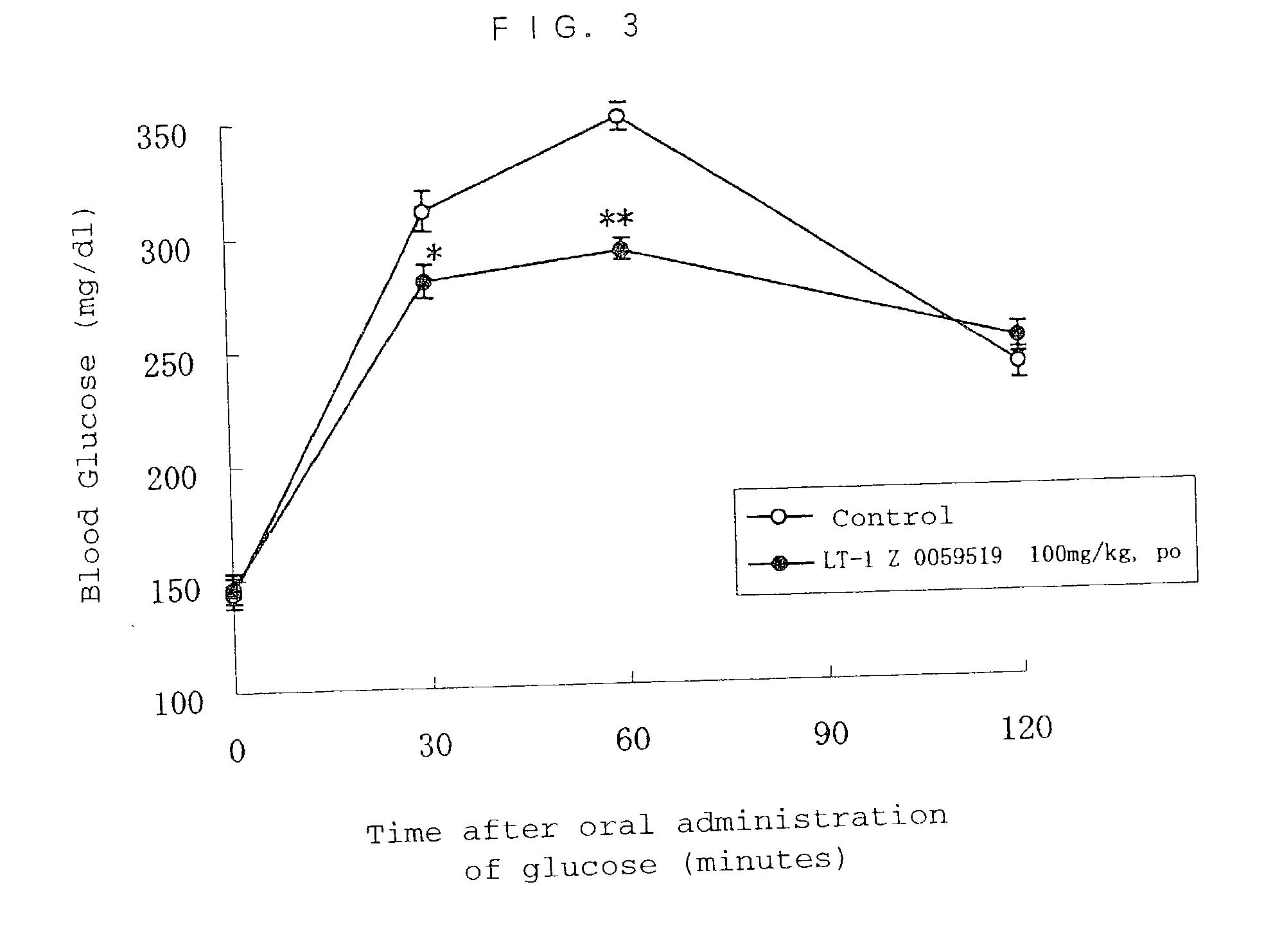
Method of screening remedy for diabetes
a screening remedy and diabetes technology, applied in the field of diabetes screening remedy, can solve the problems of reducing blood glucose, increasing the number of patients in accordance, and affecting the effect of insulin preparations, so as to promote insulin secretion, promote insulin secretion, and promote insulin secretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation of Polynucleotide Encoding Polypeptide Consisting of Amino Acid Sequence of SEQ ID NO: 2
[0162] A full-length cDNA encoding the polypeptide consisting of the amino acid sequence of SEQ ID NO: 2 was prepared by a reverse transcriptase-polymerase chain reaction (RT-PCR), using a human genomic DNA (TOYOBO) as a template, in accordance with the following procedures.
[0163] An oligonucleotide consisting of a nucleotide sequence of SEQ ID NO: 3 was used as a forward primer, and an oligonucleotide consisting of a nucleotide sequence of SEQ ID NO: 4 was used as a reverse primer. At each of the 5'-termini of the primers, an XbaI recognition sequence added thereto existed, respectively. The RT-PCR was carried out, using a polymerase (Pyrobest DNA polymerase; Takara-shuzo) in the presence of 5% dimethylsulfoxide (DMSO), by repeating a cycle composed of treatments at 98.degree. C. for 10 seconds, at 58.degree. C. for 30 seconds, and at 72.degree. C. for 2 minutes, 34 times. As a result,...
example 2
Confirmation of Expression Distribution of mRNA of Polypeptide Consisting of Amino Acid Sequence of SEQ ID NO: 2
[0169] An expression distribution of the polynucleotide encoding the polypeptide consisting of the amino acid sequence of SEQ ID NO: 2 was analyzed by an RT-PCR method in accordance with the following procedures.
[0170] In a first step, a poly A+ RNA (5 .mu.g; Clontech) prepared from human organs, more particularly, brain, i.e., amygdala, caudate nucleus, hippocampus, corpus callosum, substantia nigra, and cerebellum, spinal cord, pituitary gland, heart, placenta, lung, trachea, liver, kidney, pancreas, small intestine, stomach, spleen, bone marrow, thymus, thyloid gland, salivary gland, adrenal gland, mammary gland, prostate, testis, and ovary, was reacted with a DNase (DNase; Nippon Gene) at 37.degree. C. for 15 minutes. A part (4 .mu.g) of a resulting poly A+ RNA treated with the DNase was used for a reaction with a reverse transcriptase (MMLV Reverse Transcriptase; Clon...
example 3
Isolation and Confirmation of Expression Distribution of Rat Polynucleotide Corresponding to that Encoding Polypeptide Consisting of Amino Acid Sequence of SEQ ID NO: 2
[0172] A rat polynucleotide corresponding to the human polynucleotide encoding the polypeptide consisting of the amino acid sequence of SEQ ID NO: 2 and isolated in Example 1 was obtained by the following procedures.
[0173] A PCR was first carried out, using a rat genomic DNA (rat genomic DNA; Clontech) as a template and the oligonucleotide consisting of the nucleotide sequence of SEQ ID NO: 11 and the oligonucleotide consisting of the nucleotide sequence of SEQ ID NO: 12, each used in Example 2, as a primer set. In the PCR, a DNA polymerase (Pyrobest DNA polymerase; Takara-shuzo) was used, and a cycle composed of treatments at 98.degree. C. for 10 seconds, at 57.degree. C. for 30 seconds, and at 72.degree. C. for 1 minute was repeated 34 times in the presence of 5% DMSO. Then, a further PCR was carried out, using resu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com