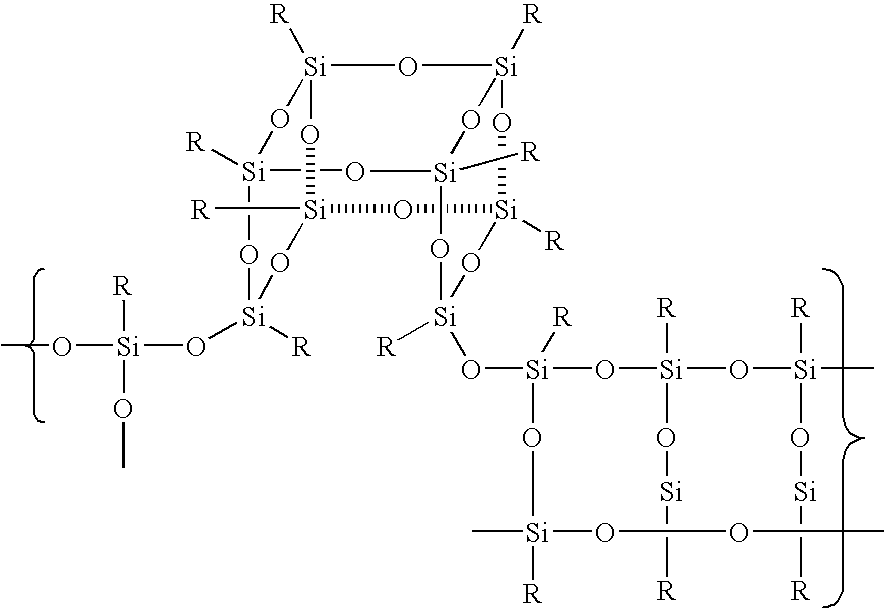
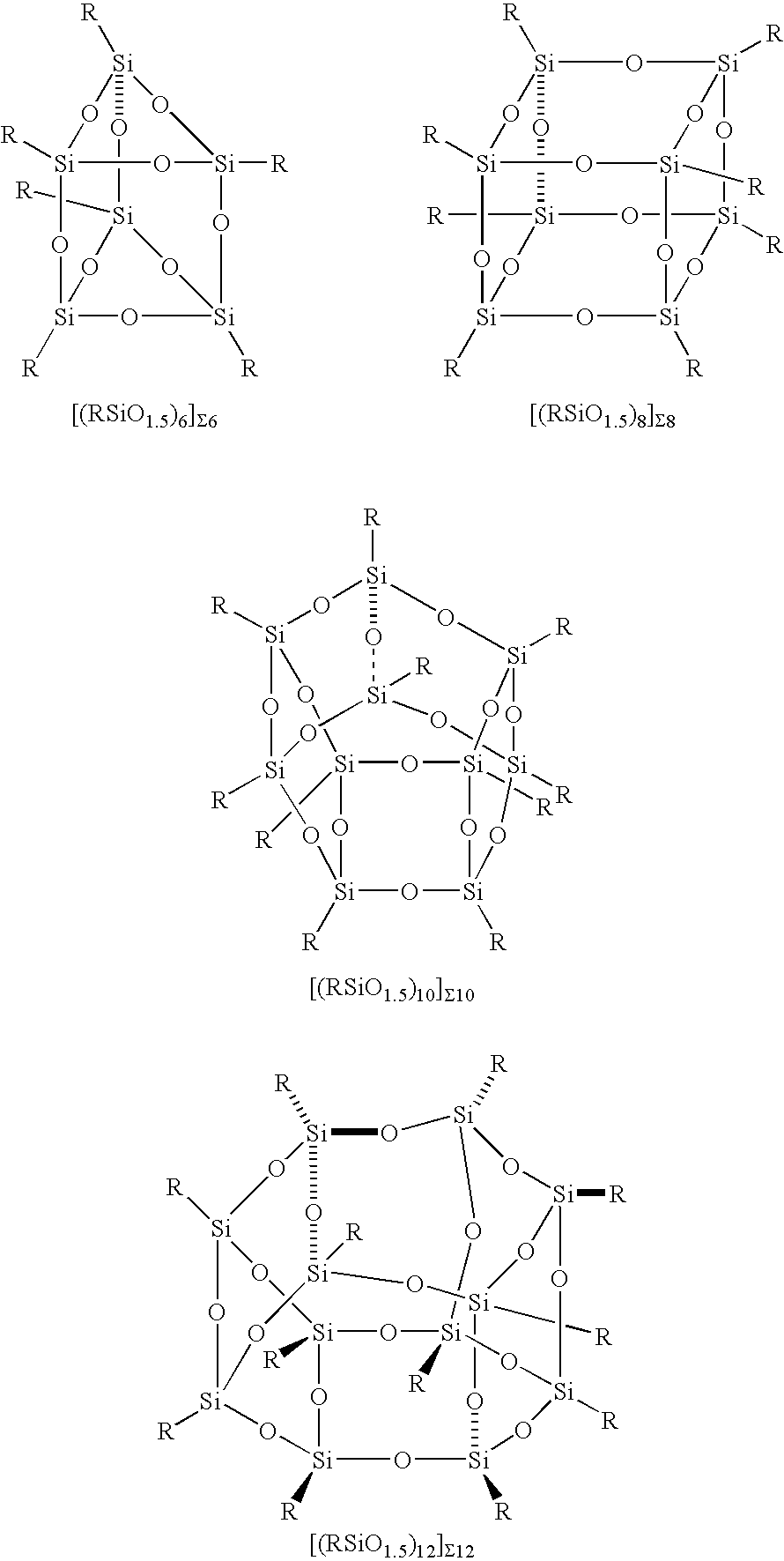
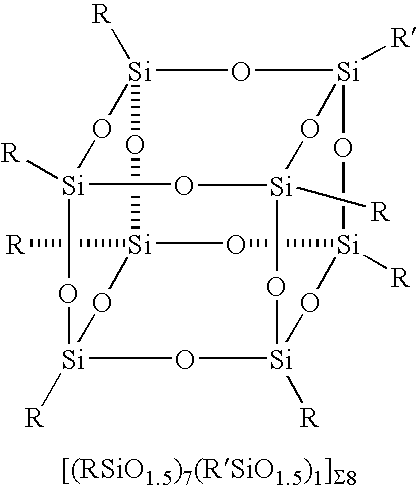
Process for the formation of polyhedral oligomeric silsesquioxanes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples for process i
The Conversion of Polysilsesquioxanes into POSS Fragments and Nanostructures
[0053] Synthesis of [((C6H5)SiO1.5)8]Σ8 from [(C6H5)SiO1.5]∞ resin. Tetramethylammonium hydroxide (2.0 mL, 5.57 mmol) was added to [(C6H5)SiO1.5]∞ resin (13.0 g, 100.6 mmol) in toluene (100 mL) at room temperature. The reaction mixture was heated to 80° C. for 12 hours, then cooled to room temperature, acidified with 1N HCl, and filtered to give 12.065 g of [((C6H5)SiO1.5)8]Σ8 as a white solid. Product was verified by EIMS which shows a molecular ion at 1032.5 amu along with daughter ions corresponding to loss of one, two, and three phenyl groups, respectively, at 954.7, 877.4, and 800.6 amu. The above procedure can be modified for the continuous and batch production. Alternately, benzene, acetone, and methyl ethyl ketone can also be used as solvents for this reaction in place of toluene and KOH can be used instead of tetraalkylammonium bases. In addition, phenyltrimethoxysilane can be used in place of phen...
examples for process ii
Reactions between POSS Systems and Silsesquioxane / Siloxane Fragments
[0073] Preparation of [((CH3)SiO1.5)7(CH3CH2OOC(CH2)10)SiO1.5)1]Σ8: One equivalent of ethylundecanoate triethoxysilane and seven equivalents of methyltrimethoxy silane (1.9 g) (were added dropwise to a refluxing solution of acetone (40 ml) and 1 ml of water containing 0.15 equivalents, 235.6 mg) of potassium acetate. The reaction was refluxed for 3 days cooled and the white crystalline product was collected via filtration and was washed with MeOH to remove resin. The product was characterized by MS and X-ray diffraction. A similar procedure was followed for each of the following compounds: [0074] [((CH3)SiO1.5)6(CH3(CH2)7)SiO1.5)2]Σ8, [((CH3)SiO1.5)7(CH2═CH)SiO1.5)1]Σ8, [0075] [((CH3)SiO1.5)4(CH2═CH)SiO1.5)4]Σ8, [((CH3)SiO1.5)6(CH2═CH)SiO1.5)2]Σ8, [0076] [((CH3)SiO1.5)7(H2N(CH2)3)SiO1.5)1]Σ8, [((C6H5)SiO1.5)7((CH2═CH)SiO1.5)1]Σ8, [0077] [((CH3)SiO1.5)7(H2N(CH2)3)SiO1.5)1]Σ8, [((c-C5H9)SiO1.5)7((CH3CH2OOC(CH2)10)SiO...
examples for process iii
Selective Opening, Functionalization and Rearrangement of POSS Nanostructures
[0100] Preparation of [((CH2═CH)SiO1.5)6((CH2═CH)(HO)SiO1.0)2]Σ8 from [((CH2═CH)SiO1.5)8]Σ8: An aqueous solution of NEt4OH (33%, 2 mL, 0.25 mmol) in THF (10 mL, −35° C.) was added to a stirred solution of [((CH2═CH)SiO1.5)8]Σ8 (2.95 g, 4.66 mmol) in 1:1:1 THF / CH2Cl2 / isopropanol (300 mL), which was chilled in a −35° C. (1:1 methanol / water and N2) cold bath. After 4.3 hours the reaction was quenched with 1M HCl (20 mL, −35° C.) and the solution was washed with 1M HCl (2×40 mL), water (2×40 mL), and sat. aq. NaCl solution (40 mL). After drying over Na2SO4, and removal of the solvent in vacuo (25° C., 0.01 Torr) a white solid (3.01 g, 99%) was isolated. The product [((CH2═CH)SiO1.5)6((CH2═CH)(HO)SiO1.0)2]Σ8 prepared by this procedure is spectroscopically pure. Additional purification can be accomplished through recrystallization from CH2Cl2 / hexanes / acetic acid (25° C.). 1H NMR (CDCl3, 500.2 MHz, 25° C.): δ 6.1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Substance count | aaaaa | aaaaa |
| Substance count | aaaaa | aaaaa |
| Substance count | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com