Ophthalmic drug delivery system using polymer micelle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(Preparation of Fluorescent-Labelled Polymer Micelle)
[0037] A diblock copolymer having polyethylene glycol (PEG) as a hydrophilic polymer chain, and polyaspartic acid (P(Asp)) as an anionic polymer chain within one molecule was dispersed in water, and mixed with FITC-labelled polylysine (FITC-P(Lys)) to prepare a core-shell type PIC micelle solution (5 mg / mL) having a core of a polylon complex (PIC) consisting of P(Asp) and FITC-P(Lys), and a shell of PEG.
(Production of Choroidal Neovascular (CNV) Model)
[0038] After generally anesthetizing a BN rat by intramuscular administration of 1 mL / kg of a mixture of a 5% ketamine hydrochloride injection and a 2% xylazine hydrochloride injection (7:1), eye drops of a 0.5% tropicamide-0.5% phenylephrine hydrochloride ophthalmic solution were administered to render mydriasis. Then, photocoagulation was carried out with a semiconductor laser photocoagulator. Photocoagulation was carried out on six scattering positions per one eye in a poster...
example 2
[0044] Incorporation property into cells was examined using dendrimer-type porphyrin (DP) that is a photosensitive substance as a drug.
(Preparation of Polymer Micelle Incorporating DP)
[0045] Polymer micelle incorporating DP was prepared according to Example 1 described in Japanese Patent No. 3422481 (the same applied to in the following Examples).
[0046] DP used herein is an anionic porphyrin dendrimer [32(−)(L3)4PZn] described in Example 1 in Japanese Patent No. 3422481 (hereinafter, referred to as DPZn).
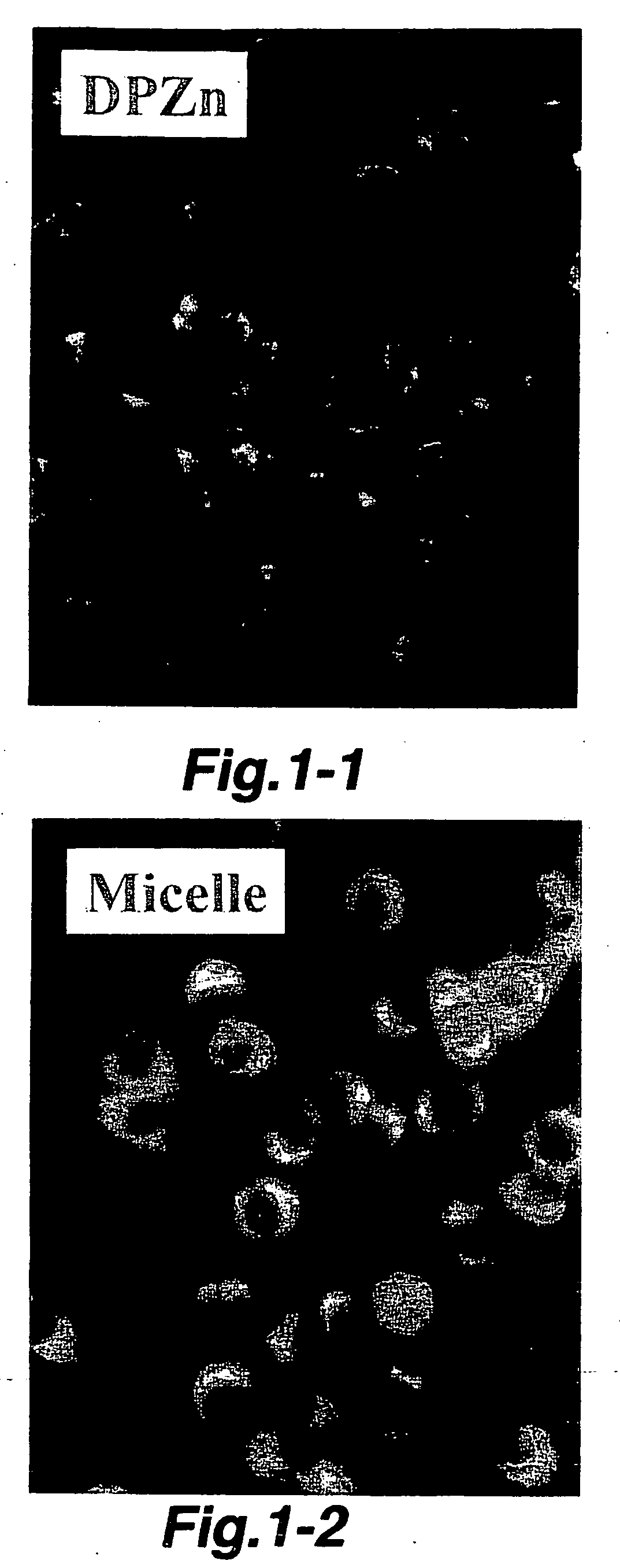
(Test on Incorporation Property into Cells)
[0047] The polymer micelle incorporating DPZn (hereinafter, referred to as DPZn / polymer micelle), and LLC (Lewis Lung Carcinoma) cells were incubated in phosphate buffered saline in a dark place at 37° C. for 8 hrs. After washing with a phosphate buffered saline, incorporation into the cells was qualitatively observed by a fluorescence microscope.
[0048] As a comparative control, DPZn and LLC cells were incubated under the same condi...
example 3
[0050] Using DPZn as a drug, accumulating capability to CNV was examined.
(Method of Administration)
[0051] The DPZn / polymer micelle was intravenously administered in a rat in which CNV was developed according to Example 1.
(Method of Evaluation and Results)
[0052] Following the administration eyeball was removed at a predetermined time. A frozen tissue section was prepared, and then accumulation to CNV was qualitatively observed by a fluorescence microscope. Consequently, as shown in FIG. 2, high accumulating capability was found agreeing with the CNV site at 0.25 hour, 1 hour, 4 hours and 24 hours after the administration.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle diameter | aaaaa | aaaaa |
| Particle diameter | aaaaa | aaaaa |
| Therapeutic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com