Solid formulations of liquid biologically active agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0106] PVP-PDLLA (POLYMER 1 and POLYMER 2) samples were dissolved in mixtures of water and various amounts of tert-butyl alcohol (TBA). Propofol is then added to the PVP-PDLLA solution. Water is then added to the TBA / PVP-PDLLA / propofol solution to the desired final volume. Final TBA concentration in these solutions was 10-30%. Drug loading levels, % w / w of propofol / (propofol+PVP-PDLLA), were also varied from 5, 7, 8, 10, 12, 15 and 20%. Solutions were then frozen in a dry ice / acetone bath and lyophilized for at least 24 hours. Lyophilized cakes obtained were then reconstituted by adding water to obtain an aqueous solution of propofol 1% w / v in less than 30 seconds. Overall results indicated that at 10% w / w drug loading levels and below, solutions were 100% homogenous. At drug loading levels above 10% w / w, the solutions were gradually more and more opalescent (bluish tint caused by diffracted light). At 20%, solutions are cloudy, but stable (no precipitation for more than 8 hours).
example 2
[0107] PVP-PDLLA (POLYMER 1) is dissolved directly in water at concentrations between 100 to 350 mg / mL. Propofol is added to the PVP-PDLLA solution and mixed until a homogenous solution is obtained. The solution is then diluted to a concentration of 1% w / v of propofol. 7, 10 and 12% w / w drug loading levels were tested. All solutions were then filtered using 0.2 μm sterile filters and frozen in acetone / dry ice bath or in −80° C. freezer for at least 4 hours before being lyophilized for 48 hours. Solid lyophilized cakes of 7, 10 and 12% w / w were reconstituted by adding water for injection. 7 and 10% w / w drug loading levels yield homogenous solutions, while the 12% w / w yielded a slightly opalescent solution (bluish tint). All where stable for more than 8 hours, i.e. no precipitation or phase separation under visual observation.
TABLE 2Reconstituted formulation characteristics of example 1.ParticleSample IDDLL theo (%Osmolalitysize1FR041124w / w)DLL exp (%)mOsm(nm)POLYMER 176.743823 (99%...
example 3
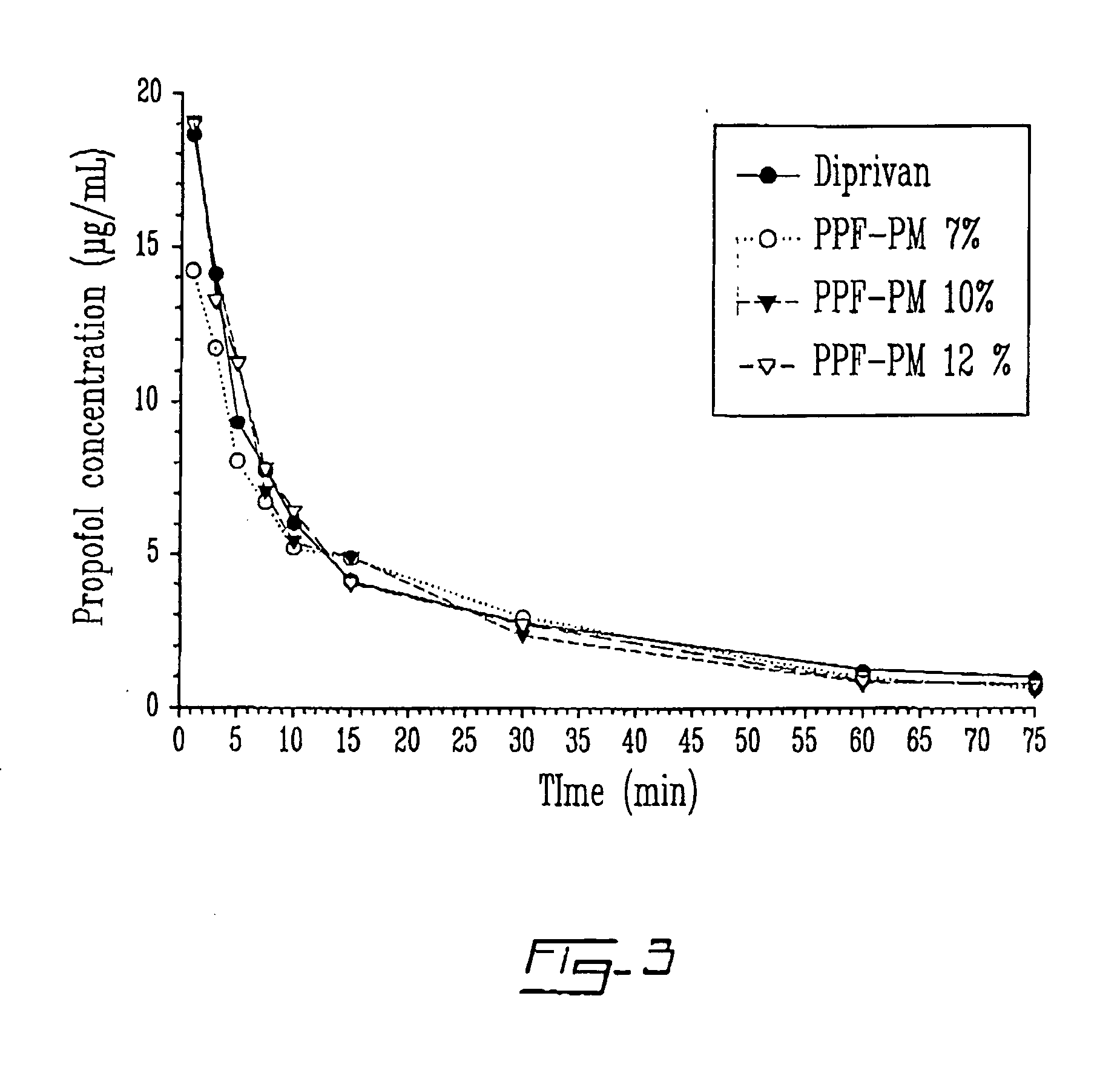
[0108] Formulations found in table 2 were tested in female Sprague-Dawley rats at a dose of 10 mg / kg. Injection time was 1 minute. All formulations prepared had a propofol concentration of 1% w / v, i.e. 10 mg / mL.
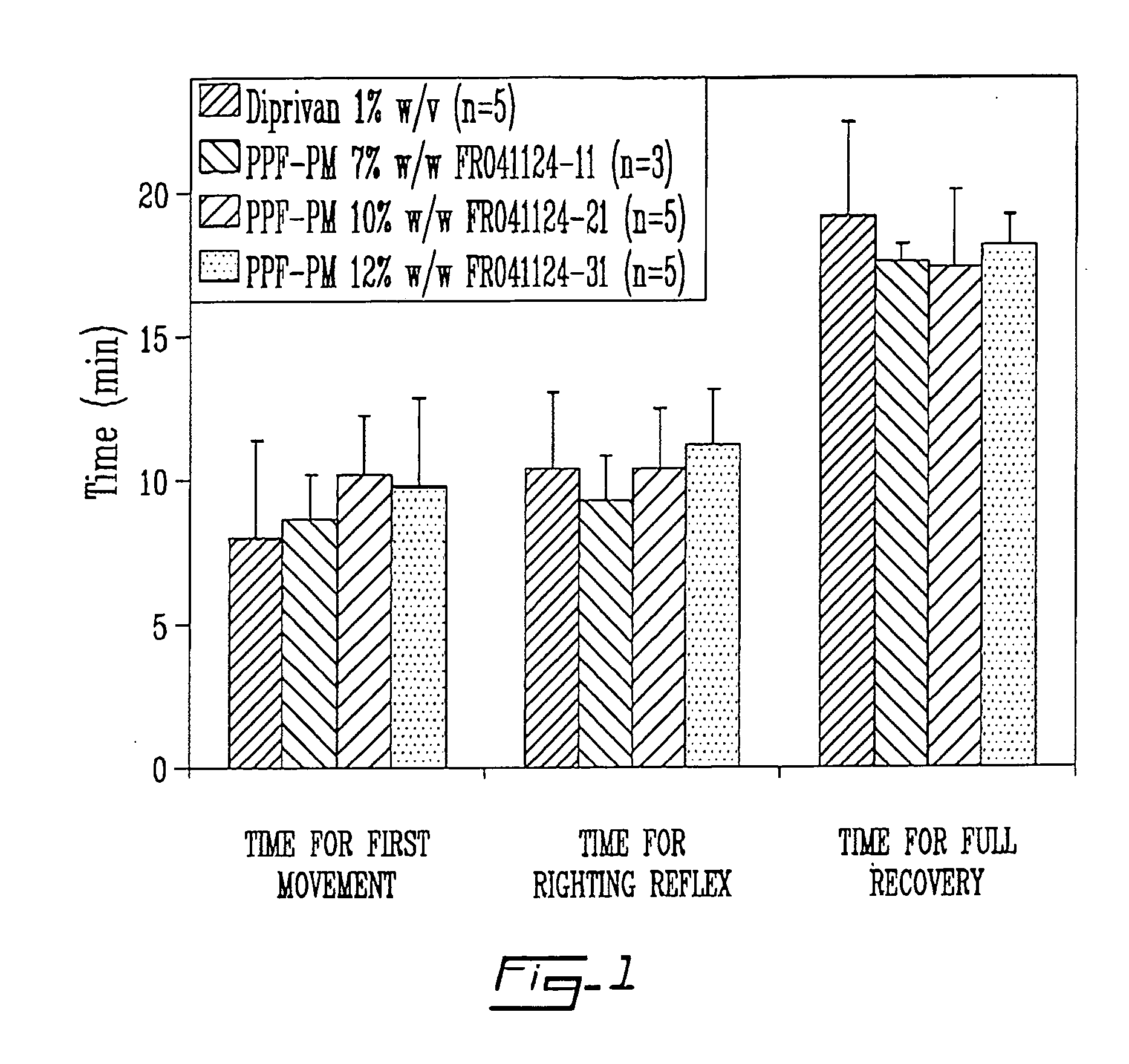
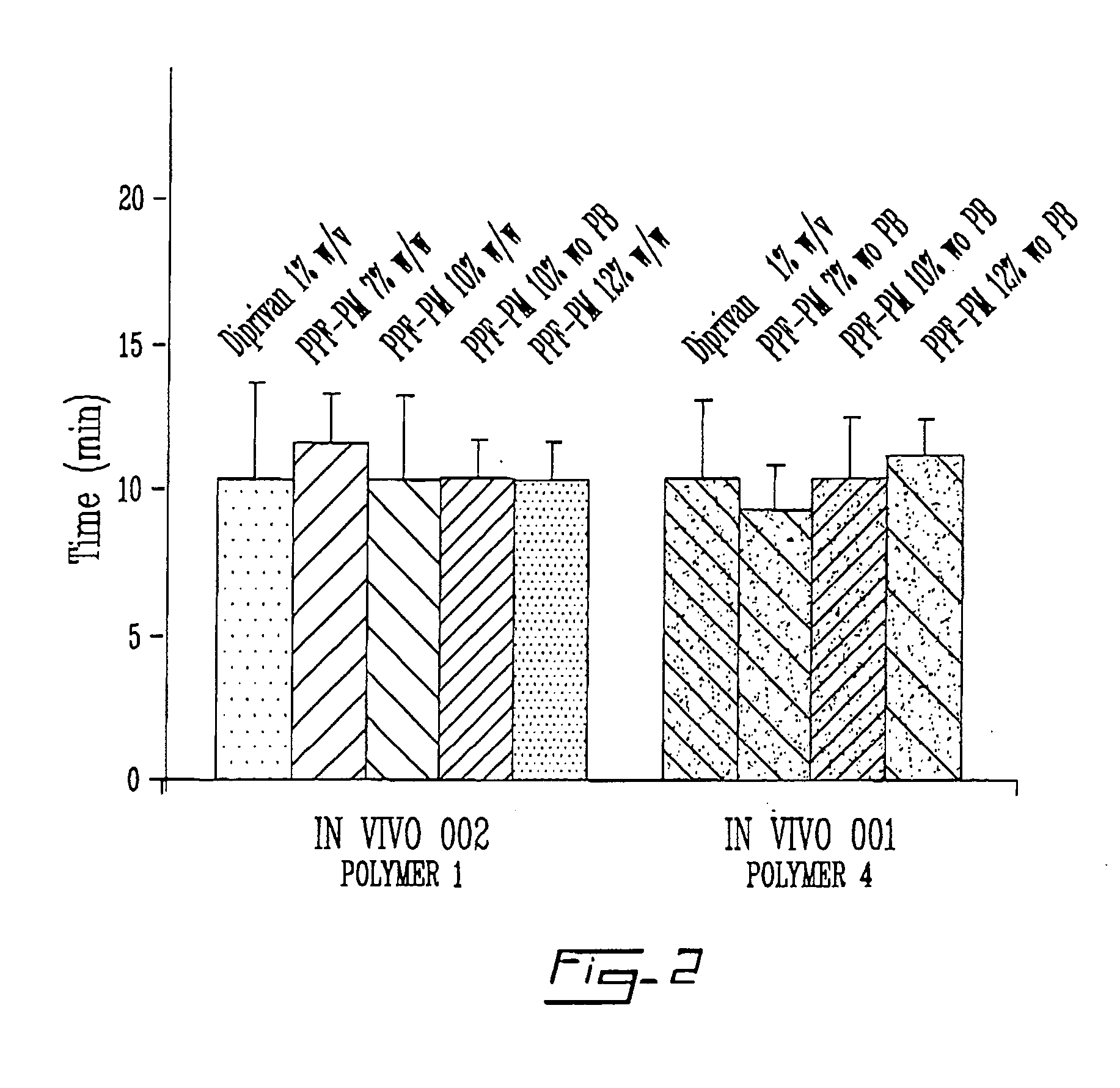
TABLE 3Pharmacodynamic parameters of Diprivan versus three propofol polymericmicelle formulations in Sprague-Dawley rats.Time of FirstTime ofMovementRightingTime of FullFormulation(min ± StdReflex (min ± StdRecovery (min ± Std(n = 5)% DLLOnset of SleepDev)Dev)Dev)Diprivan ®ca. 7% 8 ± 3.410.4 ± 2.719.2 ± 3.3FR041124-11 7%8.7 ± 1.5 9.3 ± 1.517.7 ± 0.6FR041124-2110%10.2 ± 2 10.4 ± 2.117.4 ± 2.7FR041124-3112%9.8 ± 3.011.2 ± 1.918.2 ± 1.1
The results of the above study are illustrated in FIG. 1 which is a sleep / recovery study upon iv administration of 10 mg / kl of propofol formulation in rats (onset of sleep less than 1 min).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com