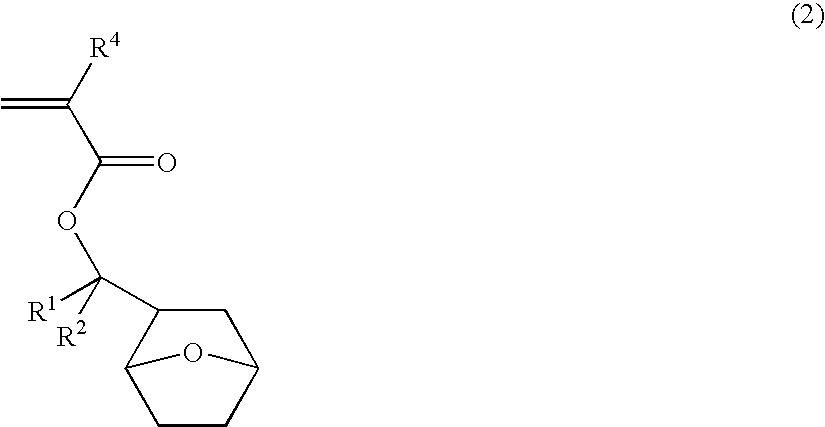
Novel ester compounds, polymers, resist compositions and patterning process
a technology of resist composition and ester compound, applied in the direction of photosensitive materials, instruments, photomechanical equipment, etc., can solve the problems of high rate of dissolution in unexposed areas, significant dimensional changes between exposure, and no acid eliminatable protective group is deemed to exert satisfactory performance, etc., to achieve the effect of reducing the dependence on line density and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
[0153] Ester compounds were synthesized in accordance with the following formulation.
synthesis example 1-1
Synthesis of Monomer 1
[0154] A flask was charged with 13.6 g of magnesium and 300 ml of tetrahydrofuran, to which 60.3 g of 1,4-dibromobutane was added dropwise at 50° C. After the completion of dropwise addition, the solution was stirred at 60° C. for one hour. To the solution below 40° C., 31.0 g of ethyl tetrahydrofurancarboxylate was added dropwise. The solution was stirred at room temperature for one hour, after which an aqueous solution of ammonium chloride was added for hydrolysis. Ordinary post-treatment yielded 30.2 g of 1-(2-tetrahydrofuranyl)cyclopentanol.
[0155] In 80 ml of toluene were dissolved 16.8 g of 1-(2-tetrahydrofuranyl)cyclopentanol, 13.1 g of triethylamine, and 0.5 g of 4-(N,N-dimethylamino)pyridine. Then 10.7 g of acrylic chloride was added to the solution at 50° C., which was stirred at the temperature for one hour. Water, 50 ml, was added to the solution below 30° C., followed by ordinary post-treatment. Vacuum distillation yielded 18.1 g of 1-(2-tetrahyd...
synthesis example 1-2
Synthesis of Monomer 2
[0156] A flask was charged with 240 ml of a solution of 1M methylmagnesium chloride in tetrahydrofuran, to which 15.6 g of methyl 7-oxa-2-norbornanecarboxylate was added dropwise below 40° C. The solution was stirred at room temperature for one hour, after which an aqueous solution of ammonium chloride was added for hydrolysis. Ordinary post-treatment yielded 14.8 g of 2-(7-oxanorbornan-2-yl)-2-propanol.
[0157] In 80 ml of toluene were dissolved 12.5 g of 2-(7-oxanorbornan-2-yl)-2-propanol, 12.1 g of triethylamine, and 0.4 g of 4-(N,N-dimethylamino)pyridine. Then 9.1 g of acrylic chloride was added to the solution at 50° C., which was stirred at the temperature for one hour. Water, 50 ml, was added to the solution below 30° C., followed by ordinary post-treatment. Vacuum distillation yielded 13.8 g of 2-(7-oxanorbornan-2-yl)-2-propyl acrylate. The two-step yield was 82%.
boiling point: 86-88° C. / 40 Pa
IR (thin film): ν=2978, 2950, 2873, 1720, 1635, 1467, 14...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com