Process For Producing In Yeast Empty Viral Capsids Consisting Of Proteins Derived From Pvp2 Of The Infectious Bursal Disease Virus (Ibdv)
a technology production process, which is applied in the field of production process of empty viral capsids consisting of proteins derived from pvp2 of infectious bursal disease virus, to achieve the effects of avoiding the handling of highly infectious materials, high yield and low economic cos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Obtaining VLPs-pVP2* by means of the expression of various regions of the pVP2 protein in yeasts
[0066] 1.1 Obtaining VLPs-pVP2-465 by means of the expression of the 1-456 region of the pVP2 protein in yeasts
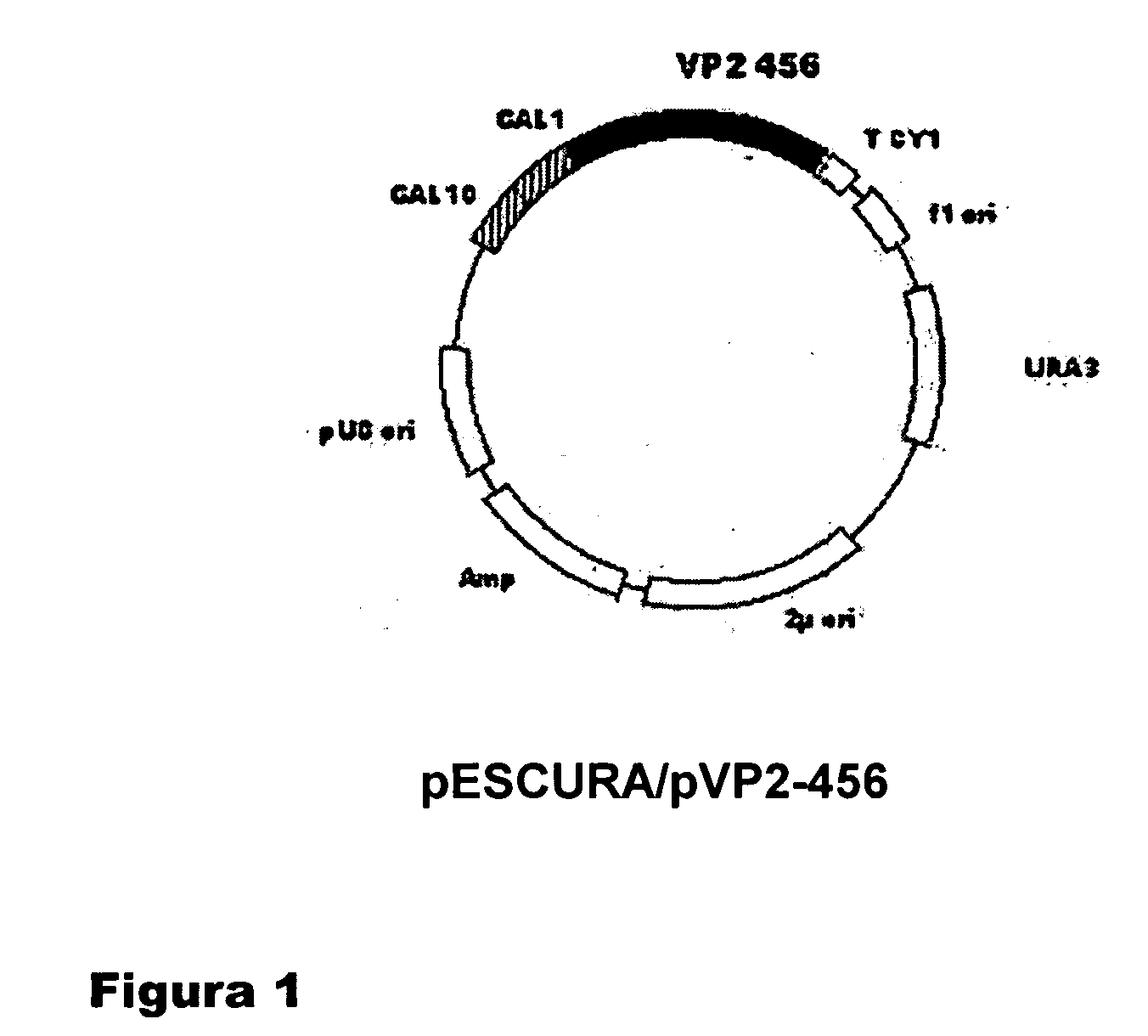
[0067] For the purpose of studying the possibility of obtaining VLPs of IBDV formed by self-assembly of the pVP2-456 protein, the amino acid sequence of which consists of the amino acid sequence comprised between residue 1 and residue 456 of the pVP2 protein of IBDV, called VLPs-pVP2-456, in yeast cultures (Saccharomyces cerevisiae), the vector pESCURAinv / pVP2-456 was generated. The first step in the construction of the vector was carried out by means of cloning the encoding region of the pVP2-456 protein (residues 1-456 of the pVP2 of IBDV) in the vector pESCURAinv. The plasmid pESCURAinv was generated by means of digesting the vector pRS426 (Stratagene) with the enzyme PvuII and religating the digestion mixture. The resulting vector, pESCURAinv, contains the multiple cloning ...
example 2
[0073] Characterization of the immunogenicity of the VLPs-pVP2-456 of IBDV
[0074] An immunization test was conducted in 1-day old chickens for the purpose of determining the immunogenicity of the VLPs-pVP2-456 (Example 1.1). A group of 7 SPF (specific pathogen free) animals was intramuscularly immunized with a single dose of 200 μl containing 10 μg of VLPs-pVP2-456 / animal diluted in PBS. A similar group was injected with PBS. Weekly serum extractions from each one of the animals of both groups were performed. The serums of each group and date were mixed to obtain a homogenous serum (pool) represented by equivalent volumes of each individual of the group. The serums were analyzed by means of ELISA. To that end, the wells were coated with 10 ng of VLPs-pVP2-456. The tests were conducted according to a previously described protocol (Current Protocols in Immunology. Edited by: Barbara Bierer, John E. Coligan, David H. Margulies, Ethan M. Shevach, Warren Strober, John Wiley & Sons http: / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| OD | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com