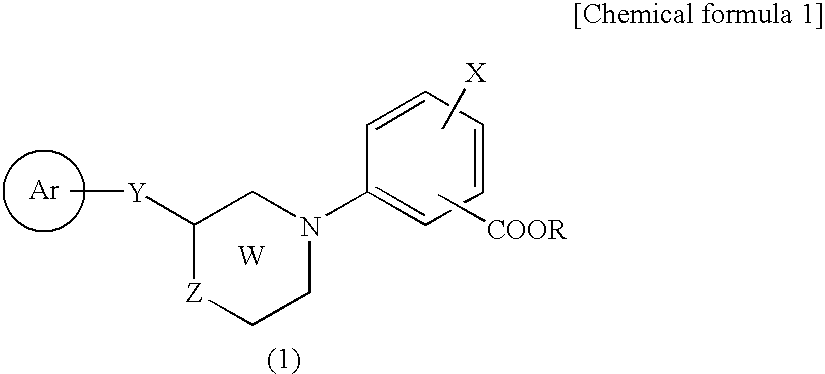
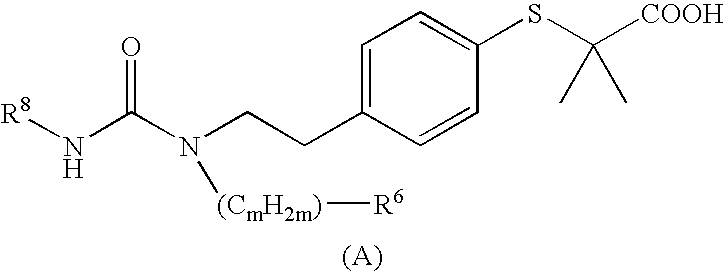
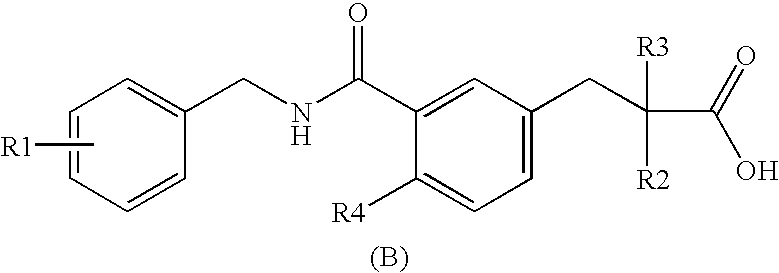
Novel Cyclic Amino Benzoic Acid Derivative
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
1-benzyl-2-oxoperhydroazepine
[0250]
[0251]ε-Caprolactum (3.00 g, 26.5 mmol) was dissolved in tetrahydrofuran (30 mL). To this solution, potassium hydride (3.34 g, 29.2 mmol) was added and the mixture was stirred at room temperature for 10 min. Benzyl chloride (3.36 mL, 29.2 mmol) and sodium iodide (100 mg) were added and the reaction mixture was further stirred at room temperature for 3 hours. Subsequently, water was added and the mixture was extracted with ethylacetate. The extract was washed with brine, dried over magnesium sulfate and concentrated. Purification of the residue by silica gel column chromatography (hexane:ethyl acetate=10:1->2:1) gave 3.04 g (57%) of the desired compound as a colorless oil.
[0252]1H NMR (400 MHz, CDCl3) δ 1.46-1.51 (2H, m), 1.66-1.74 (4H, m), 2.60-2.62 (2H, m), 3.28-3.30 (2H, m), 4.59 (2H, s), 7.25-7.33 (5H, m).
[0253] FAB+ (m / z): 204 (M+H).
reference example 2
Methyl 1-benzyl-2-oxoperhydroazepine-3-carboxylate
[0254]
[0255] Diisopropylamine (2.95 mL, 21.0 mmol) and n-butyllithium (11.3 mL, 18.0 mmol) were dissolved in diethyl ether (50 mL) at −78° C. and the mixture was stirred for 10 min. 1-benzylperhydroazepine (3.04 g, 15.0 mmol) in diethyl ether (3 mL) was then added and the mixture was further stirred at room temperature for 10 min. The mixture was then stirred for another 10 min while bubbled with carbon dioxide. Subsequently, the reaction mixture was added to ice water and the aqueous layer was collected. 2 mol / L hydrochloric acid was added to make the mixture acidic. The mixture was then extracted with ethyl acetate and the extract washed with brine and dried over magnesium sulfate, followed by evaporation of the solvent. The resulting colorless oil (2.97 g) was dissolved in a 10% hydrogen chloride / methanol solution and the mixture was stirred at room temperature for 2 hours. The mixture was then concentrated. Water was added to th...
reference example 3
1-Benzyl perhydroazepin-3-yl methanol
[0258]
[0259] Methyl 1-benzyl-2-oxoperhydroazepine-3-carboxylate (2.39 g, 9.15 mmol) was dissolved in tetrahydrofuran (50 mL). To this solution, lithium aluminum hydride (868 mg, 18.3 mmol) was added and the mixture was stirred for 2 hours while being refluxed. Subsequently, ice water and a 10% aqueous sodium hydroxide solution were added and the mixture was stirred at room temperature for 1 hour. The reaction mixture was then filtered through Celite. The filtrate was extracted with ethyl acetate and the extract washed with brine, followed by drying over magnesium sulfate and evaporation of the solvent. Purification of the residue by silica gel column chromatography (Chromatorex NH-DM2035 (Fuji Sylysia Chemical Co., Ltd.) hexane ethyl acetate=20:1->3:1) gave 1.30 g (65%) of the desired compound as a colorless oil.
[0260]1H NMR (400 MHz, CDCl3) δ 1.49-1.65 (3H, m), 1.70-1.86 (4H, m), 2.22-2.29 (1H, m), 2.70-2.80 (3H, m), 3.45 (1H, dd, J=10.4, 4.3 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com