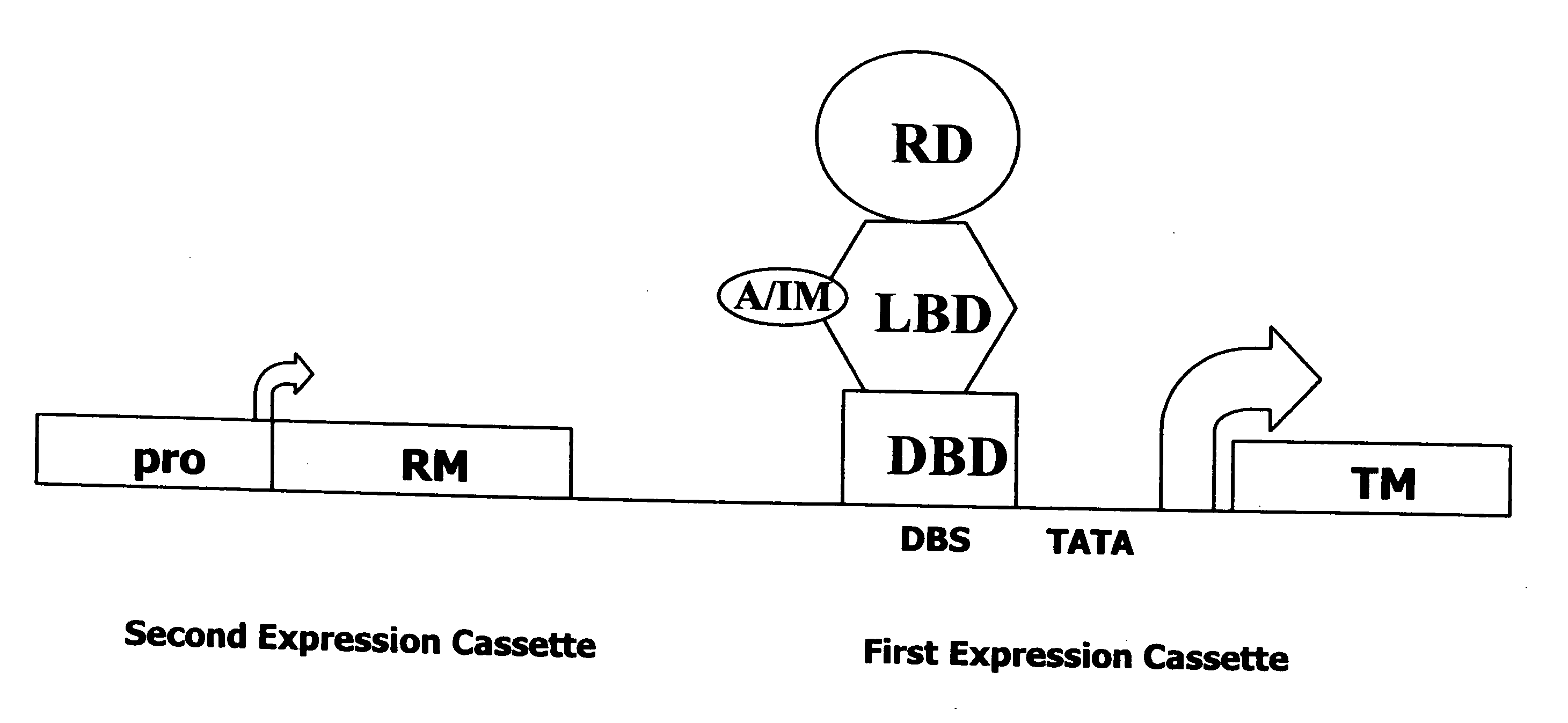
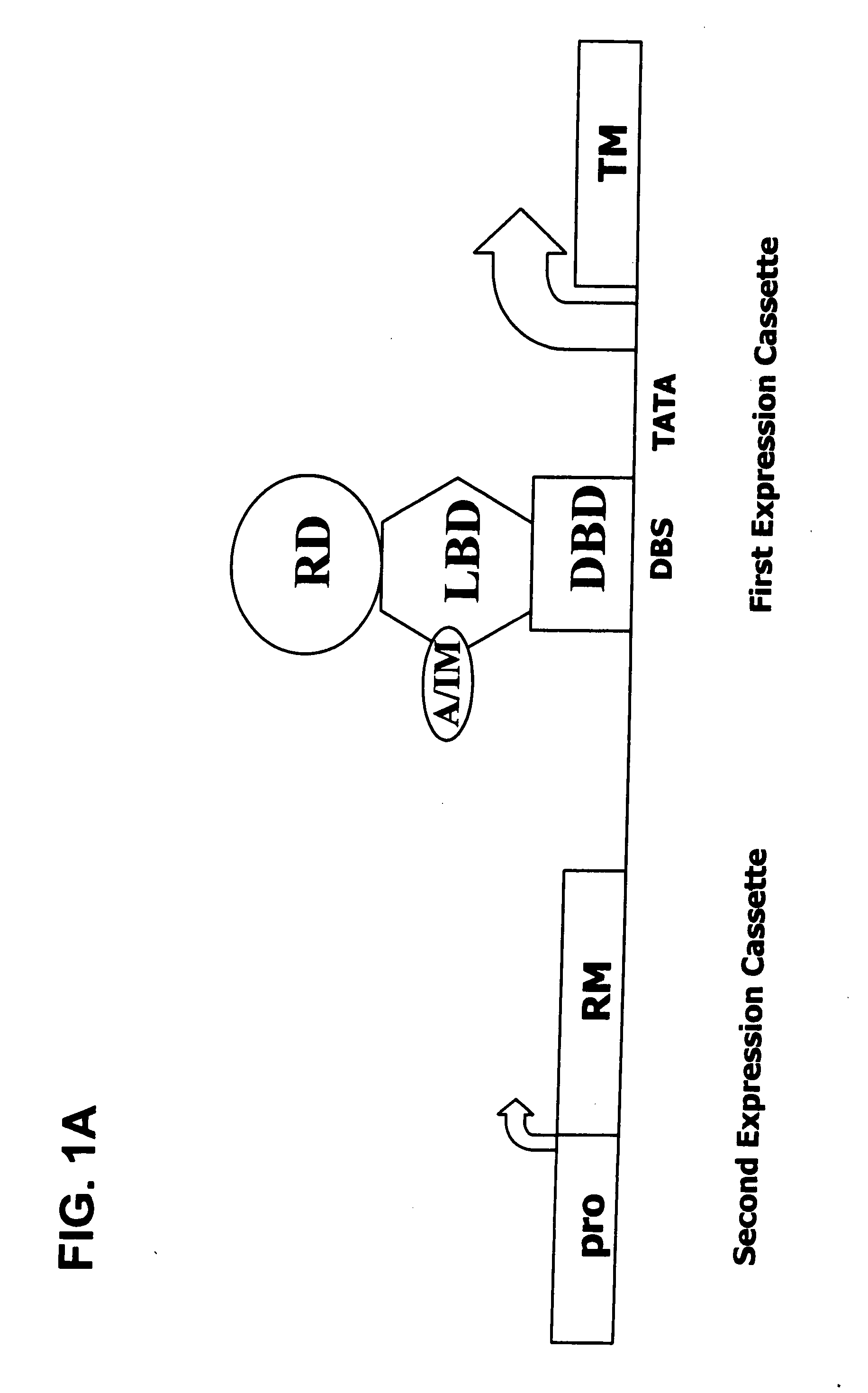
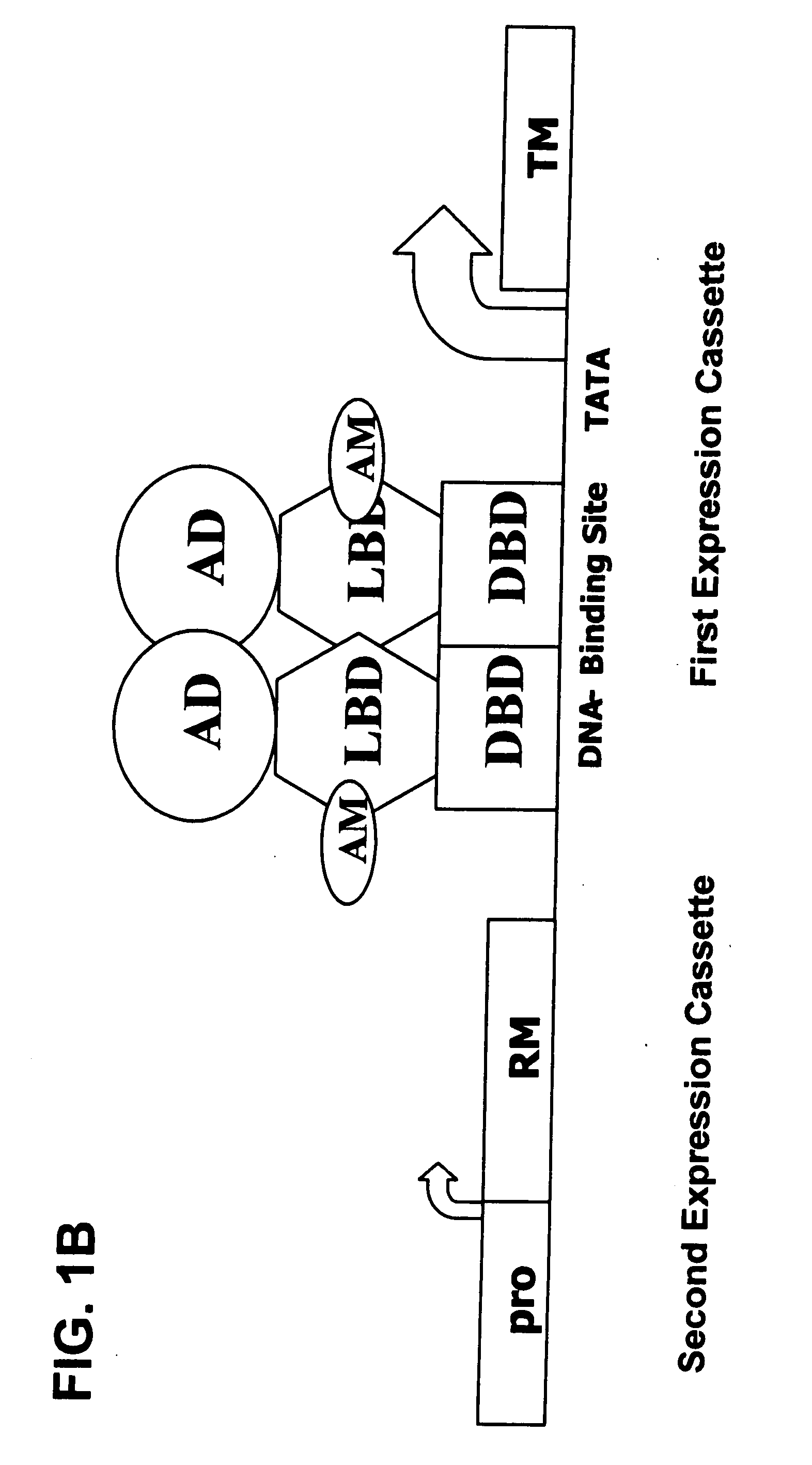
Interferon-beta gene therapy using an improved, regulated expression system
a gene therapy and interferon technology, applied in the direction of genetic material ingredients, drug compositions, peptides, etc., can solve the problems of affecting the effect of tm, and reducing the therapeutic effect of tm, so as to maximize the therapeutic effect and minimize the side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Vectors for Use in IFN-β or GMCSF Gene Therapy
[0365] A. Plasmid Vectors: The murine IFN-β (mIFN-β) gene from the bacterial expression vector pbSER189 was PCR amplified, with immunoglobulin kappa (IgK) (for protein purification) or mIFN-β (for gene therapy) signal sequence added on the 5′ primer. The PCR products were inserted downstream of the cytomegalovirus (CMV) promoter in the expression vectors pCEP4MWPRE, to generate pGER90 (FIG. 2A) for recombinant protein expression and purification, and pgwiz, to generate pGER101 (FIG. 2B) for gene therapy. [0366] The human IFN-β gene from the bacterial expression vector pbSER178 was PCR amplified by the same procedure as the mIFN-β gene (except with the hIFN signal sequence for the gene therapy vector) and inserted into pCEP4 / WPRE to generate pGER123 (FIG. 2C) for recombinant protein expression and purification, and pgwiz to generate pGER125 (FIG. 2D) for gene therapy. [0367] The construction of plasmid vectors is fully de...
example 2
Pharmacokinetic Studies of IFN-β Gene Delivery
[0371] A. Pharmacokinetic Studies with Human IFN-β: Pharmacokinetic studies were performed in normal mice to compare bolus protein versus gene-based delivery of human IFN-β (hIFN-β). [0372] 1) Human IFN-β1a Protein Phamacokinetic Study: A pharmacokinetic study was carried out in C57BL / 6 mice using bolus injection of recombinant hIFN-β1a delivered either by intramuscular (i.m.) or intravenous (i.v.) injection and using a commercially available ELISA to detect serum levels of hIFN-β. FIG. 3 shows the pharmacokinetic profile of hIFN-β1a protein in serum of mice following a single i.m. or i.v. injection of either 25 ng (1 ug / kg) or 250 ng (10 ug / kg) of hIFN-β1a protein. Following i.v. injection, hIFN-β1a was detected in serum in a dose dependent manner at the first time point (30 min), and was rapidly cleared such that the levels were near the limit of detection (LOD) of the assay (LOD=12.5 pg / ml) by 6 hours. Following i.m. injection of rec...
example 3
Identification and Use of IFN-β Biomarkers for Gene Therapy
[0374] A. Development of mIFN-β Biomarkers: For higher sensitivity in detection of murine IFN-β (mIFN-β) activity in vivo, biomarkers for mIFN-β activity were identified in mice after injection of mIFN-β protein or mIFN-β encoded gene therapy vectors. Biomarkers can be used to follow human IFN-β activity in clinical samples from patients treated with Betaseron (IFN-β 1b) (see e.g., Arnason, B G (1996) Clin Immunol Immunopathol 81: 1-11; Deisenhammer, F et al. (2000) Neurology 54: 2055-60; Knobler, R L et al. (1993) J Interferon Res 13: 333-40.; Kracke, A et al. (2000) Neurology 541: 193-9). One of the primary biomarkers used in the IFN-β clinical studies is MxA (see e.g., Kracke, A et al. (2000) Neurology 541: 193-9; Bertoloto, A et al. (2001) J Imm Meth 256: 141-152) since it is specifically induced by type I IFN's (see e.g., von Wussow, P et al (1990) J Imm 20:2015-19). In the present study, the expression of the MxA mous...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com