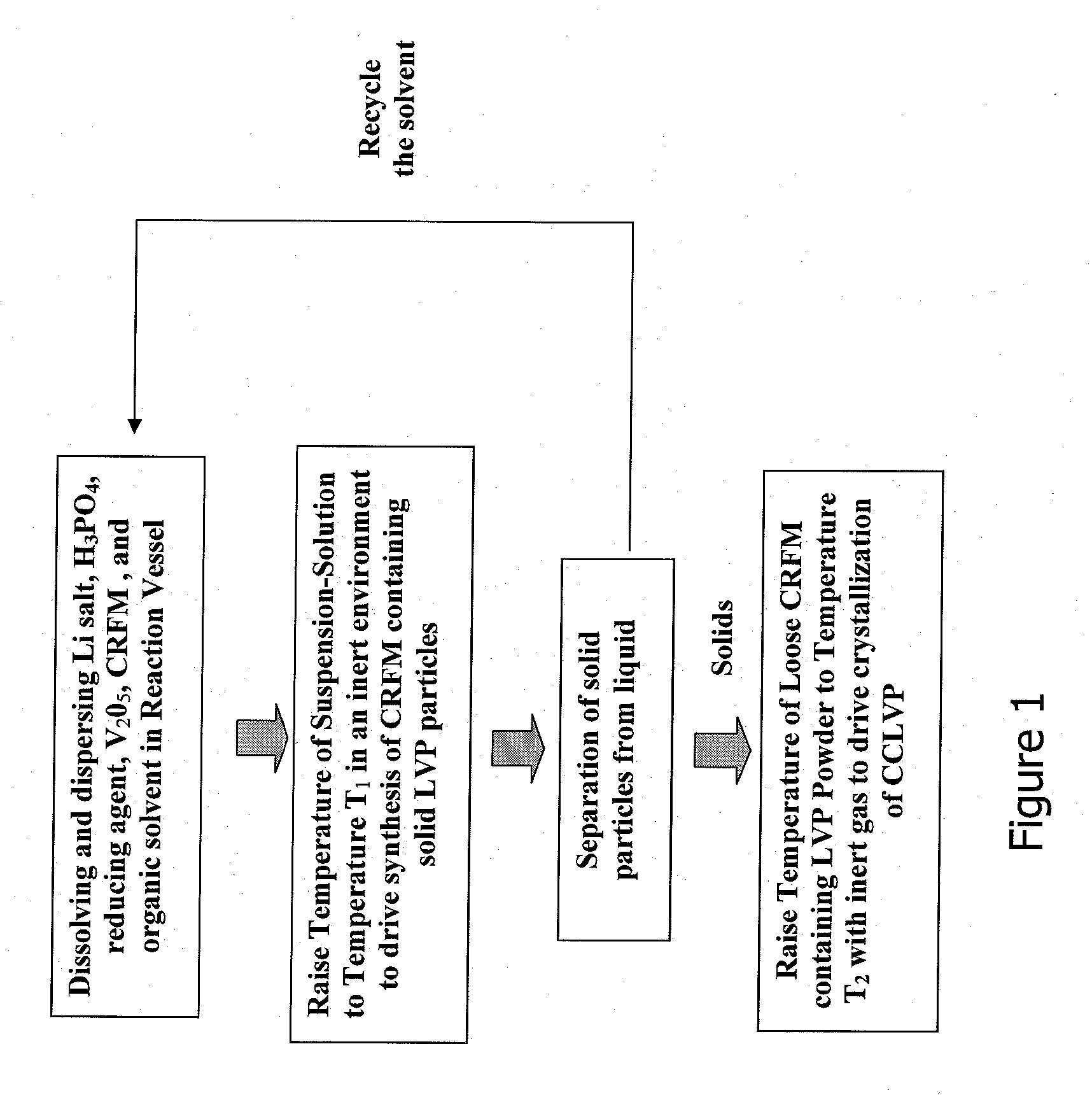
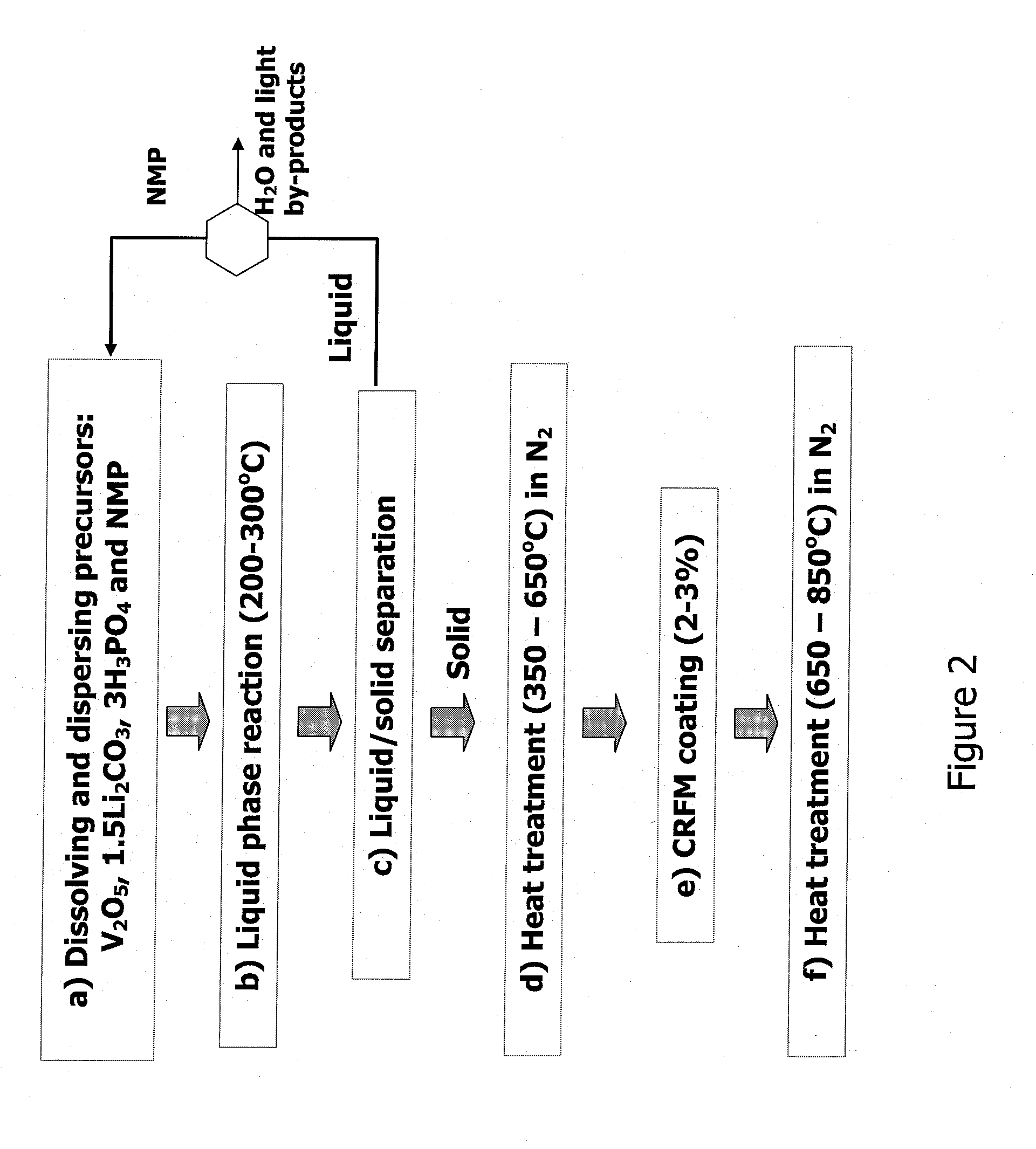
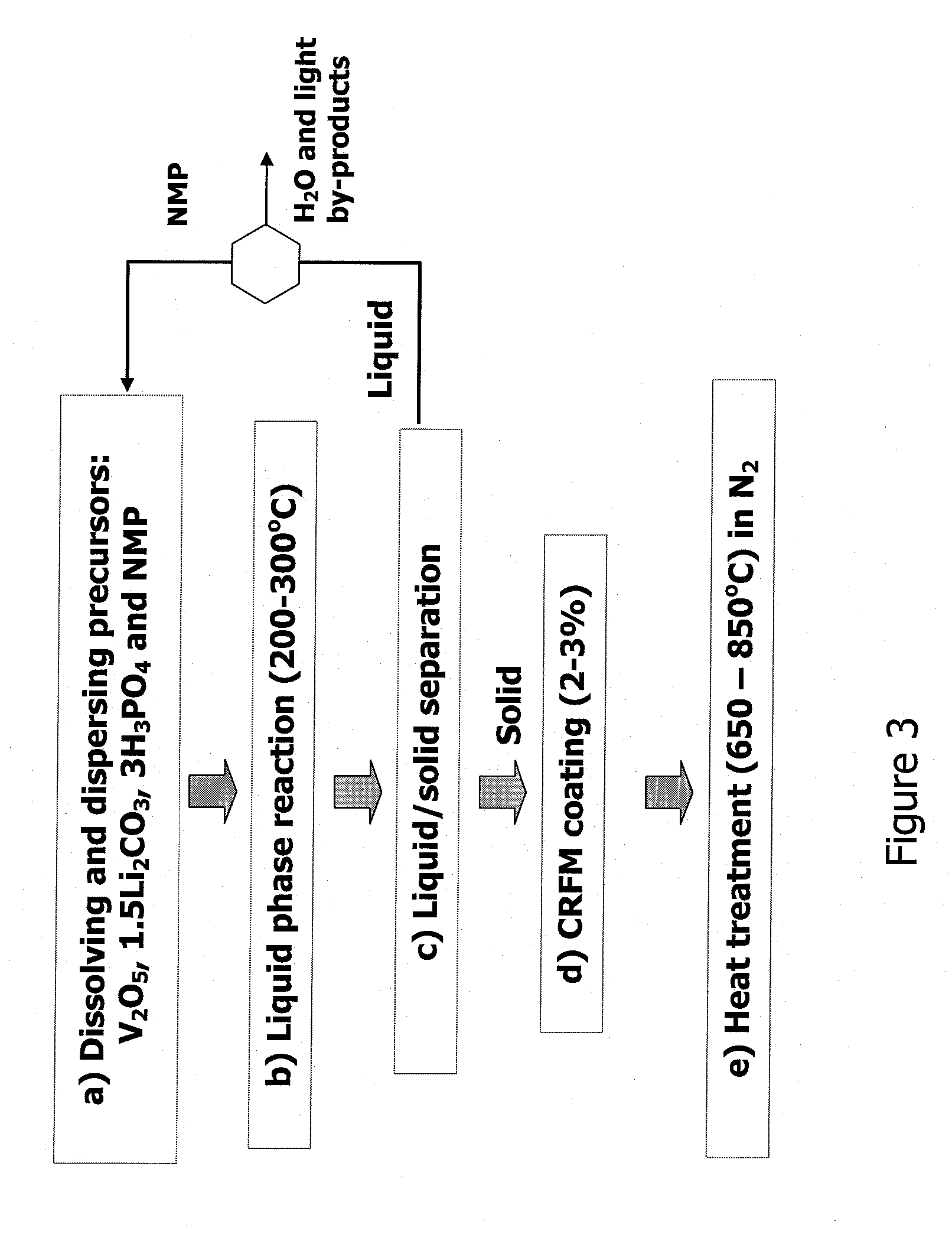
Method for producing lithium vanadium polyanion powders for batteries
a technology of lithium vanadium and polyanion powder, which is applied in the direction of lithium compounds, lithium compounds, rubidium/caesium/francium compounds, etc., can solve the problems of low energy density of cobalt and nickel, unsafe cobalt performance, and inability to provide the energy density of other transition metals such as cobalt and nickel, used commercially, etc., to achieve good electrical conductivity, improve electrical conductivity, and ensure the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0054]9.27 grams of V2O5 powder (99.2%, Alfa Chemical) were ball-milled with 150 ml of NMP for about 10 minutes, and subsequently transferred into a beaker. 17.3 grams of 86% phosphoric acid (H3PO4) were slowly poured into the beaker while the suspension was stirred continuously. 5.547 grams of lithium carbonate (Li2CO3) were then slowly added into the beaker while it was stirred continuously. The resulting solution / suspension contained solid vanadium pentoxide and dissolved lithium hydrogen phosphate. 1.5 grams of a petroleum pitch were dissolved in the suspension. The resulting suspension was transferred into a 500 ml stainless steel pressure vessel, 7.5 g of n-butanol (CH3(CH2)3OH) was subsequently added to the vessel.
[0055]The suspension was heated in the pressure vessel at 250° C. for 3 hours while the suspension was continuously agitated. The suspension was allowed to cool to room temperature. The resulting solid particles were separated from the liquid by filtration, and then...
example 2
[0057]5 grams of the sample in Example 1 was heated further at 850° C. for 6 hours in a nitrogen gas atmosphere. The resulting powder weighed 4.91 g, and remained as a loose flowable powder. The carbon content and electrochemical properties of Example 2 are given in Table 1 below.
example 3
[0058]Pitch coating and carbonization—The product powder made in Example 1 was coated with pitch. First, 14.4 grams of the product powder was dispersed in xylene. Then, 2.20 grams of petroleum pitch were dissolved in about 2.2 grams of xylene and heated to 90° C. The pitch / xylene solution was combined with the powder / xylene suspension and the combined suspension was heated at 140° C. for 10 minutes under continuous agitation. The heat was subsequently removed to let the suspension cool to room temperature. The resulting solid powder was separated by filtration and dried at 100° C. under vacuum. The resulting powder weighed 14.8 grams, yielding about 2.8% pitch by weight.
[0059]The above pitch-coated powder was placed in a tube furnace and heated in nitrogen gas under the following sequences: the temperature was ramped up at a rate of 1° C. / minute to 250° C., held at 300° C. for 4 hours, ramped at 1° C. / m to 400° C., held at 400° C. for 2 hours, and then cooled down to room temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com