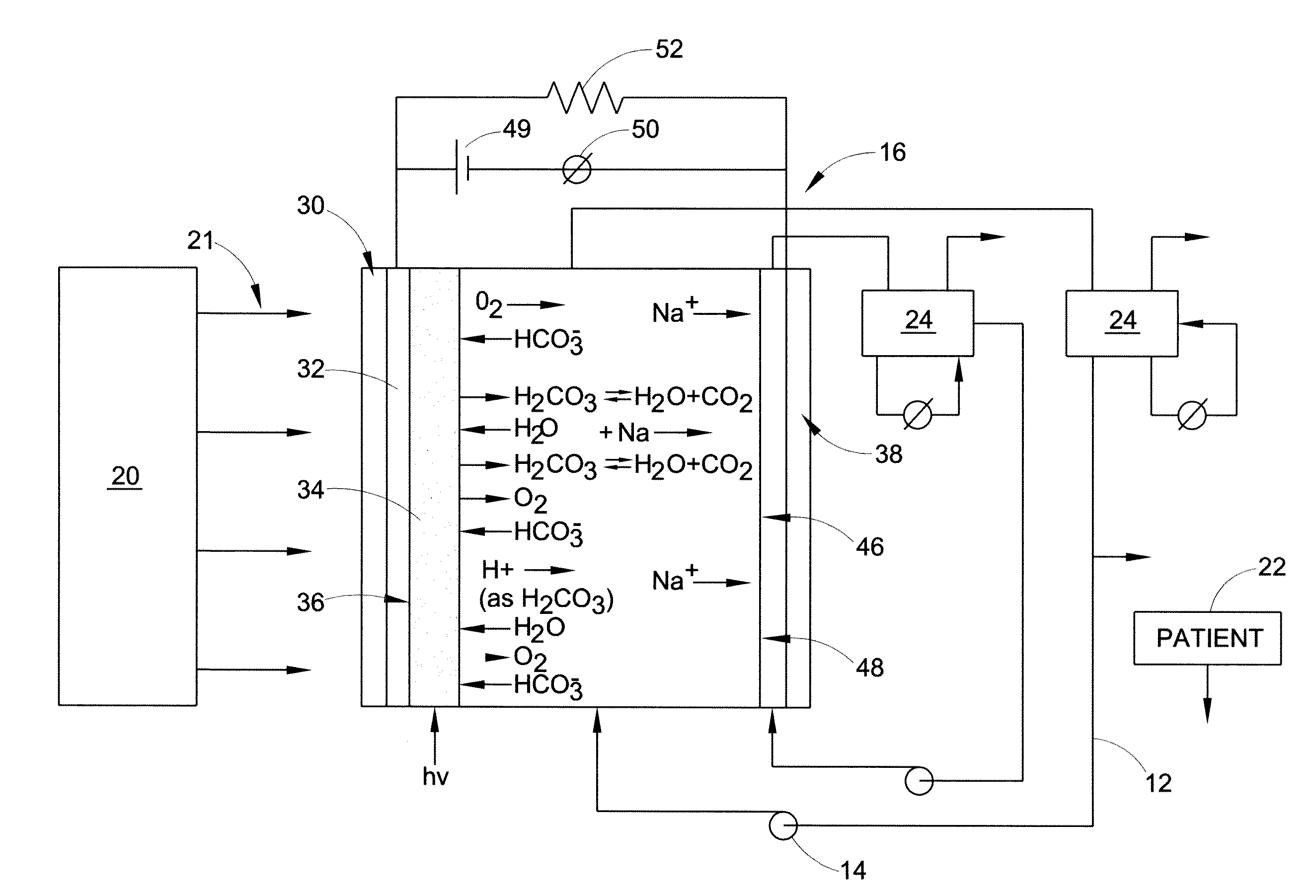
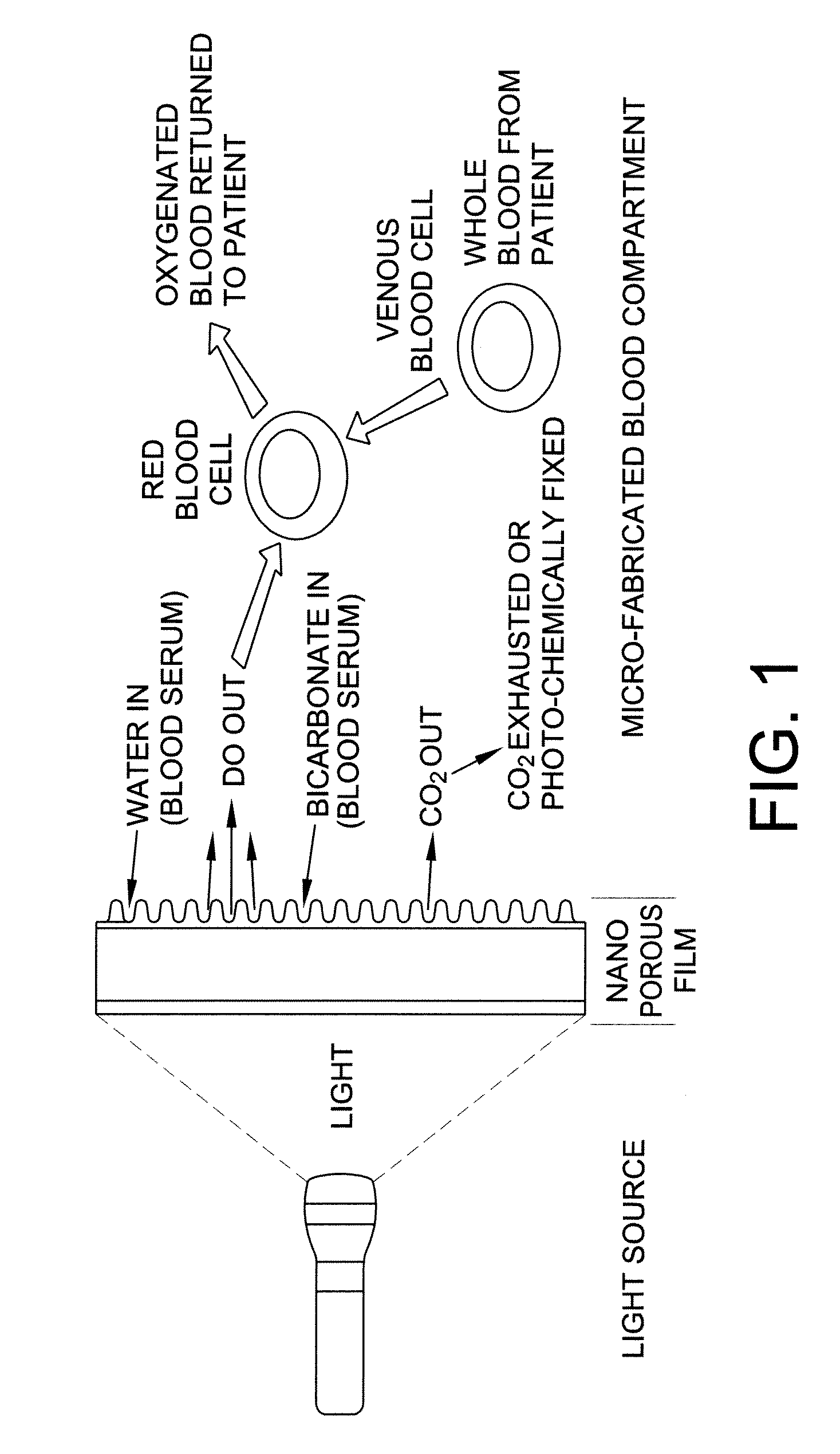
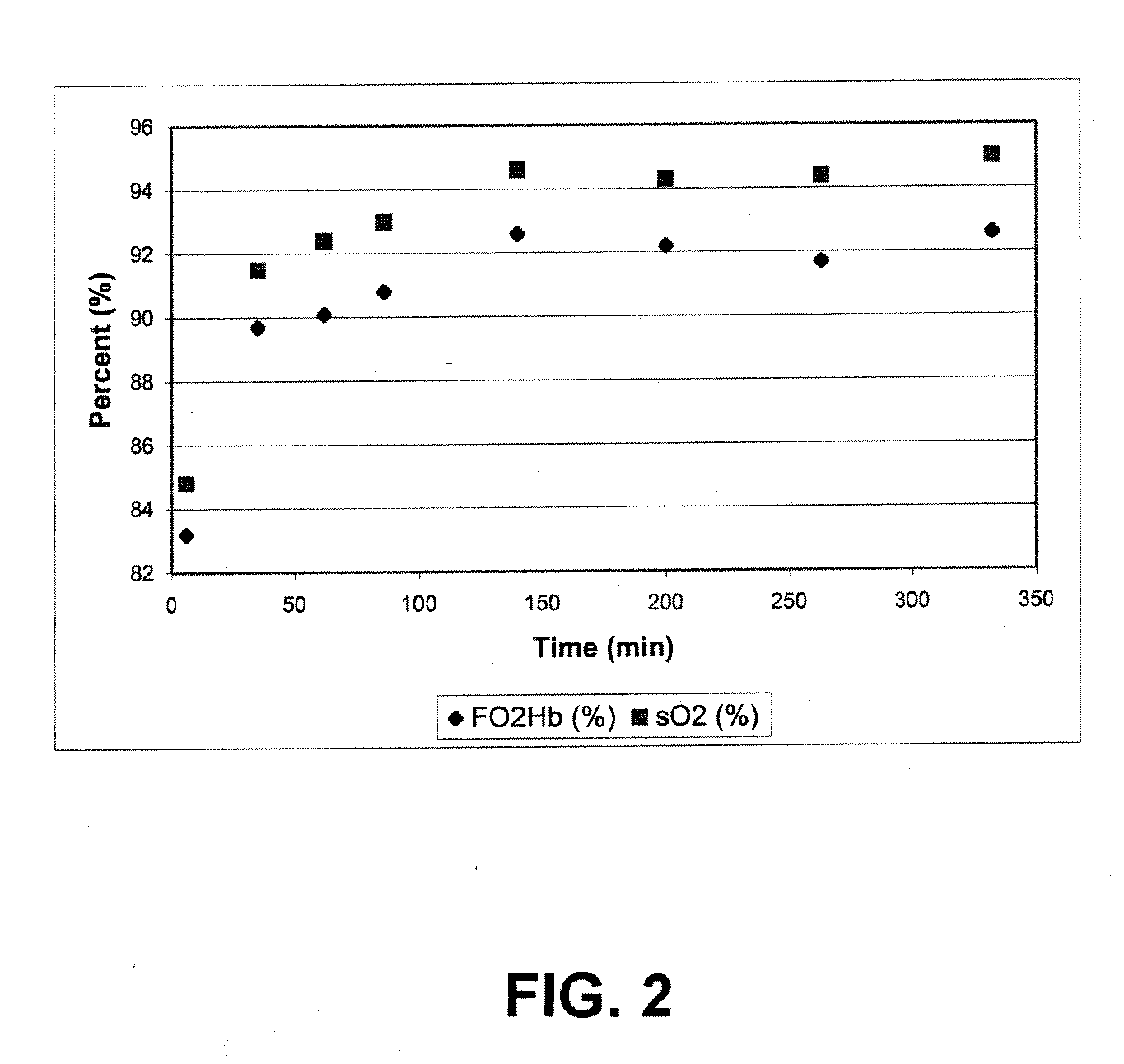
Carbon dioxide removal from whole blood by photolytic activation
a technology of photolysis and activated carbon dioxide, which is applied in the field of photolysis equipment, can solve the problems of insufficient technology, no one is believed, and insufficient yield, safety, and ease of us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0119]This example illustrates the fabrication of photoactive layers. Oxide materials were added to a substrate using a spin coating technique. Glass slides containing the conducting layer were placed on a vacuum. For the TiO2 coating, 0.5 g of the acid treated material was added to 40 ml isopropanol and mixed for 30 minutes. 0.050 ml H2O and 0.100 ml titanium(IV) tetra(isopropoxide) (TTIP), a sol-gel reagent, was added to this solution. After mixing for 30 minutes, the solution was added drop-wise to the rotating substrate for a total volume of about 12 ml. In the case of the constructs containing MnO2, following the addition of 9 ml of TiO2 slurry, 0.20 g MnO2 was added to the remaining slurry. Exactly four ml of the resulting solution was then added drop-wise to the substrate at spin coating conditions. As a modification of this technique, RuO2 / Pt doped TiO2 (0.125 g) was added to 10 ml isopropanol. After 15 minutes of mixing, 50 uL water and 25 uL TTIP was added and allowed to m...
example 2
[0120]In addition to batch cells, a flow-through test cell was constructed to associate flowing liquids (such as blood) with photolytic output. A modified FM01-LC Electrolyser, operating in a divided cell mode with a Nafion™ cation exchange membrane was used. The anode was optically transparent, and illumination was achieved side-on by UV light (354 nm) using a filter and UVA fiber optic lamp source. The catholyte was Locke's-Ringer solution, and the anolyte fresh whole bovine blood containing anticoagulant. The photolytic surface was TiO2 on a quartz plate. Fluids were maintained at 37° C. using an in-line heat exchange jacket, and flow was 80 cc / min by a peristaltic pump. The flow-through cell consisted of a 3 ml photolytic chamber, with a single active surface of vacuum deposited Ti metal, a coating of TiO2 (anatase) and optionally a MnO2 layer. These films were prepared as described above for the batch test films, except on larger glass and quartz substrates (effective area ˜5 i...
example 3
[0122]This example illustrates real-time measurement of dissolved oxygen (DO): A liquid phase in line reaction chamber was used to monitor dissolved oxygen production. This device utilized a Clark Electrode to measure dissolved oxygen (DO). A two-point calibration procedure was used to calibrate the dissolved oxygen sensor. Dasse K A, Monzyk B F, Burckle E C, Busch J R, Gilbert R J., Development of a photolytic artificial lung: Preliminary concept validation, ASAIO Journal, 48:556-563, 2003. A low bias voltage was applied to the cell through a DC power source, sufficient to insure proper anode-to-cathode electron flow direction, to promote immediate removal of photo-generated electrons, but insufficient to drive electrochemical side reactions, as evidenced by the lack of current flow in the absence of illumination. Dasse K A, Monzyk B F, Burckle E C, Busch J R, Gilbert R J., Development of a photolytic artificial lung: Preliminary concept validation, In press, ASAIO Journal, 2003. E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com