Formulation and method for the treatment of fungal nail infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Characterisation of Antimicrobial Activities in Manuka Honey—Absence of Endogenous Hydrogen Peroxide
[0208]Using the Spectrophotometric bioassay described, antimicrobial activity of commercially available Manuka honey is determined, using several samples to ensure consistency.
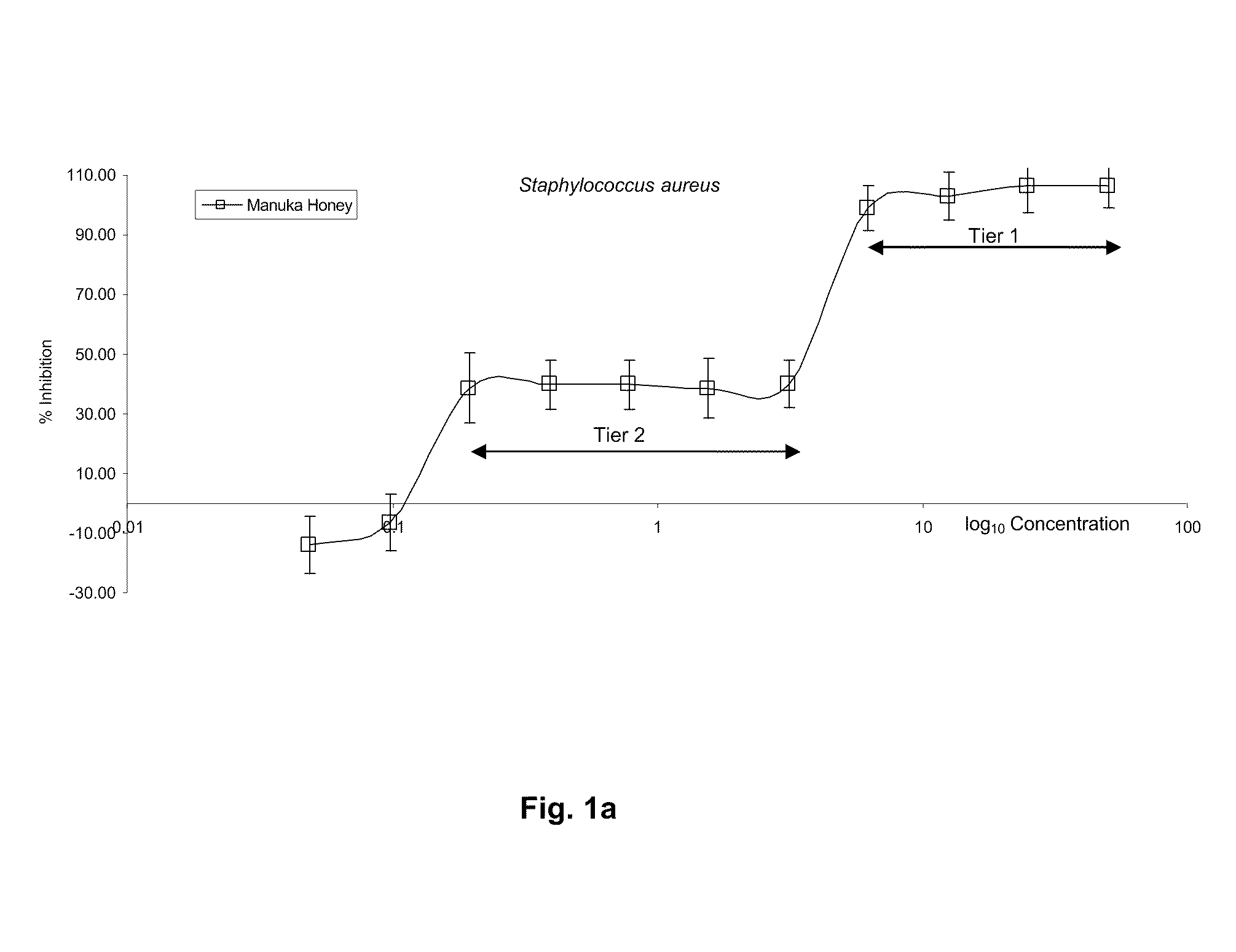
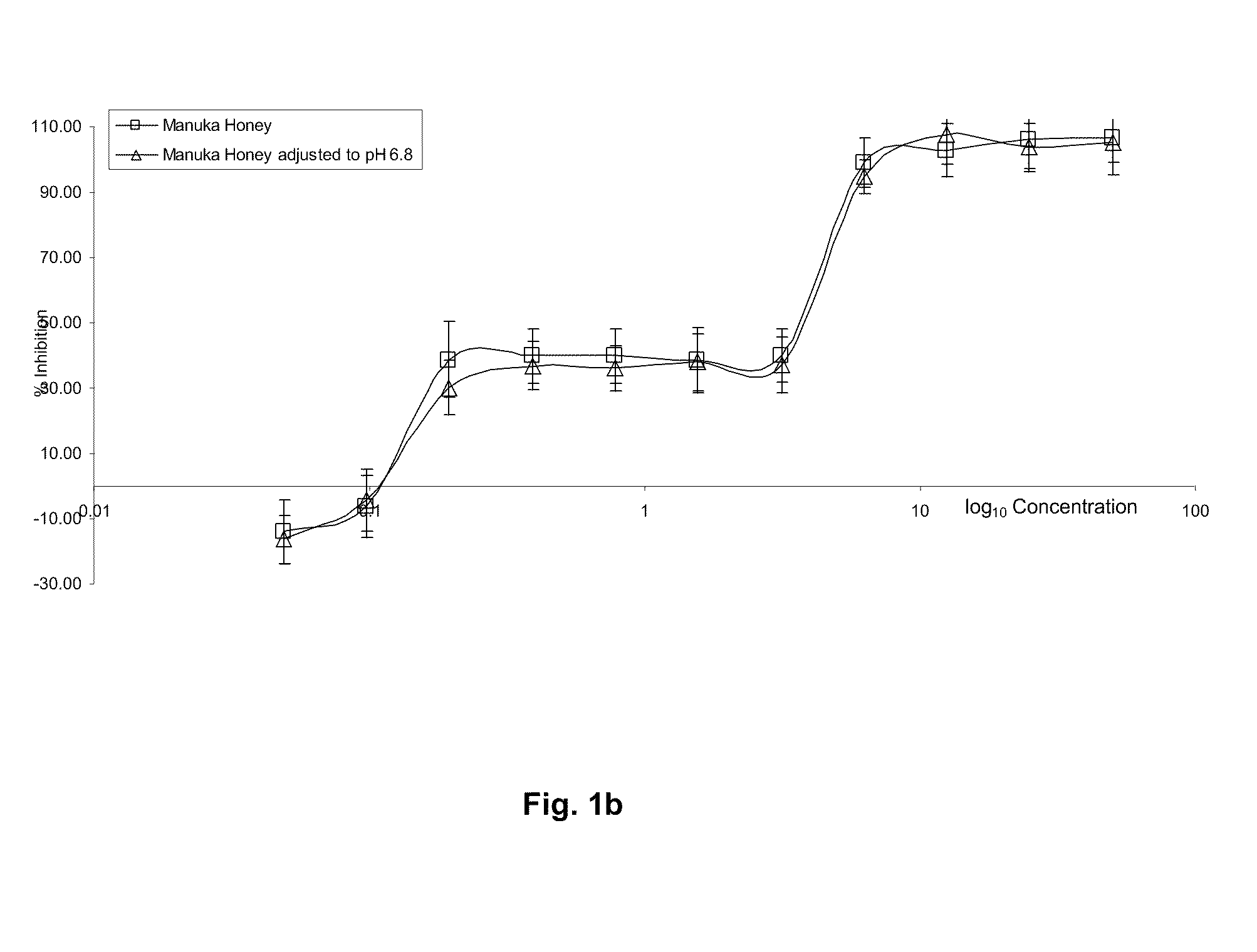
[0209]Results shown in FIG. 1a demonstrate that Manuka honey provides a first tier of microbial inhibition activity at dilutions 50% to approximately 6.25% and a second tier of microbial inhibition activity at dilutions 3.125% to approximately 0.195%
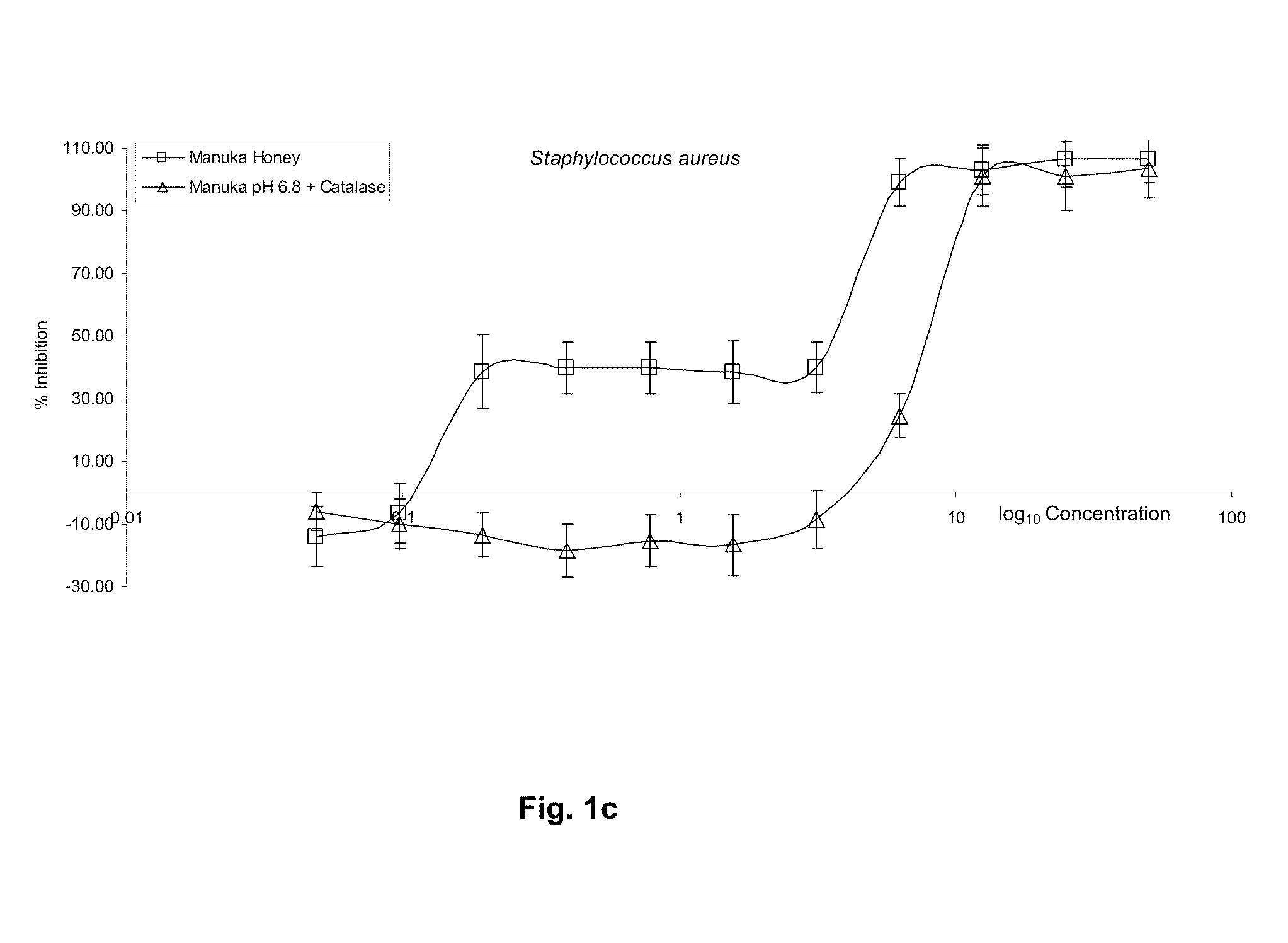
[0210]This two tier effect is shown to be produced by separate mechanisms. Initial microbial inhibition on low honey dilution (50%-6.25%) results from a combination of low pH and growth limiting Aw (Available Water) and a very minor role by hydrogen peroxide, which is only produced de-novo upon dilution and after a considerable period of time has elapsed. There is no detectable endogenous hydrogen peroxide present in diluted or undiluted Manuka honey, as shown in Tab...
example 2
A Prototype Antimicrobial Endogenous and Sustained Release Hydrogen Peroxide Generating System
[0215]A prototype formulation containing 31+ / −5 g glucose: 35+ / −5 g fructose: 7+ / −2 g maltose: 1.5+ / −1 g sucrose is made by mixing the ingredients, making the mixture up to a final volume of 100 ml in distilled deionized (DI) water; the mixture is sterilized by autoclaving. Glucose oxidase at 0.05% by weight, which is a similar concentration to that contained in Manuka honey, is added.
[0216]FIG. 2 shows the results of an antimicrobial assay on S. aureus using this prototype formulation. The prototype formulation of this example demonstrated a greater activity compared to Manuka honey. It is probable that the critical role played by the glucose oxidase enzymatic pathway in the antibacterial effect is enhanced once free from impurities and reaction limiting compounds (such as catalase) present in honey. This prototype demonstrates very effective bactericidal activity.
example 2.1
A Gel Prototype Antimicrobial Endogenous and Sustained Release Hydrogen Peroxide Generating System
[0217]Gelling agents that are common ingredients in topical pharmaceutical formulations are added to the prototype formulation and tested. Gels tested include water reconstituted cellulose and alcohol reconstituted cellulose agents (1. carbomer, 2. methocel, 3. polyvinylpyrrolidone and 4. xanthan gum at 2% in a hydrogel incorporating the prototype formulation). Both cellulose based gels demonstrate a decrease in stability. It is possible that steric hindrance and hydrolysis of the glucose oxidase result in loss of antibacterial activity. Even before loss of activity, due to decreased stability, neither gel formulations is as active as the prototype formulation, as evidenced by the smaller zones of inhibition in diffusion assays (compare FIG. 3a (gels) with FIG. 3b (prototype formulation)).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com