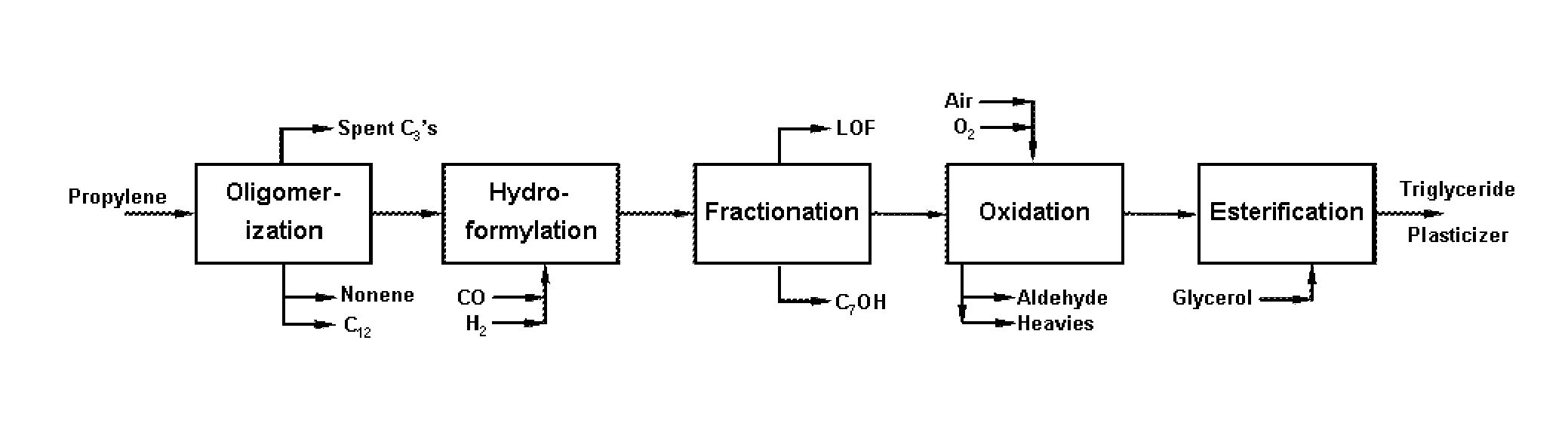
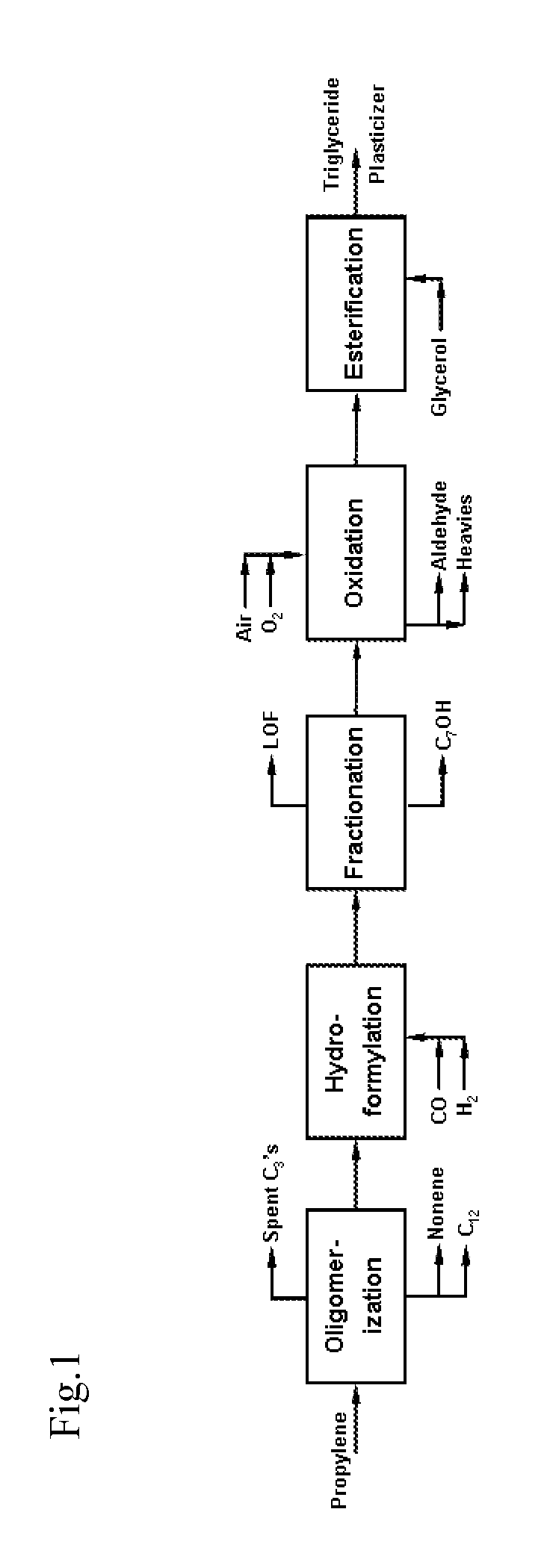
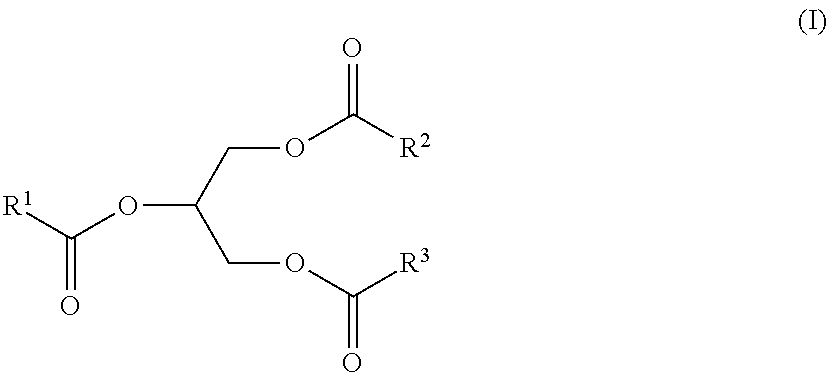
Polyol Ester Plasticizers and Process of Making the Same
a polyol ester and plasticizer technology, applied in the field of triglyceride esters, can solve the problems of cyclohexanoic acid processability, “slow fusion” plasticizers, and special effects, and achieve the effects of reducing toxicity concerns, reducing toxicity, and reducing aging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 4
[0140]A mixture comprising 100 grams of glycerol tris-isoheptanoate, 0.5 grams stearic acid, 4 grams of epoxidized soybean oil, and 5 grams of the Calcium / Zinc PVC stabilizer Mark 1221 (from Chemtura) was slowly heated to 50° C. on a hot plate, with stirring, until the stearic acid dissolved. The solution was cooled to room temperature, then slowly poured over 200 grams of PVC resin (Oxy 240) and mixed in a Hobart mixer for 5 minutes. The powder mixture was then heated on a room mill, at 165° C. for 5 minutes. The sheets from the roll mill were then compression molded at 171° C., first at 1000 psig, then at 3500 psig. After cooling the sheets were removed for testing.
[0141]The PVC specimens were conditioned for 7 days at 22° C. and 50% relative humidity before testing. The following properties were recorded for the isoC7 triglyceride ester in this PVC formulation: Shore A Hardness (15 sec) 76; tensile strength 3144 psi, elongation 394%, weight loss after 7 days at 100° C. 10.9%, car...
example 5
[0142]A mixture comprising 80 grams of glycerol tris-isoheptanoate, 0.5 grams stearic acid, 4 grams of epoxidized soybean oil, and 5 grams of the Calcium / Zinc PVC stabilizer Mark 1221 (from Chemtura) was slowly heated to 50° C. on a hot plate, with stirring, until the stearic acid dissolved. The solution was cooled to room temperature, then slowly poured over 200 grams of PVC resin (Oxy 240) and mixed in a Hobart mixer for 5 minutes. The powder mixture was then heated on a room mill, at 165° C. for 5 minutes. The sheets from the roll mill were then compression molded at 171 C, first at 1000 psig, then at 3500 psig. After cooling the sheets were removed for testing.
[0143]The PVC specimens were conditioned for 7 days at 22° C. and 50% relative humidity before testing. The following properties were recorded for the isoC7 triglyceride ester in this PVC formulation: Shore A Hardness (15 sec) 86; tensile strength 1968 psi, elongation 353%, Clashberg Tf of −22° C., and Bell Brittleness tem...
example 6
[0144]A mixture comprising 100 grams of glycerol tris-(isoheptanoate / benzoate), 0.5 grams stearic acid, 4 grams of epoxidized soybean oil, and 5 grams of the Calcium / Zinc PVC stabilizer Mark 1221 (from Chemtura) was slowly heated to 50° C. on a hot plate, with stirring, until the stearic acid dissolved. The solution was cooled to room temperature, then slowly poured over 200 grams of PVC resin (Oxy 240) and mixed in a Hobart mixer for 5 minutes. The powder mixture was then heated on a room mill, at 165° C. for 5 minutes. The sheets from the roll mill were then compression molded at 171° C., first at 1000 psig, then at 3500 psig. After cooling the sheets were removed for testing.
[0145]The PVC specimens were conditioned for 7 days at 22° C. and 50% relative humidity before testing. No exudation was observed. The following properties were recorded for the iso C7 triglyceride / benzoate tri ester in this PVC formulation:[0146]Shore A Hardness (15 sec) 74; tensile strength 2982 psi, elonga...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com