Pharmaceutical composition and method for treating hypogonadism
a technology of pharmaceutical composition and testosterone, which is applied in the field of pharmaceutical composition and method for treating hypogonadism, can solve the problems of low lh concentration, low efficacy of high lh concentration in stimulating testosterone production, and affecting the libido of men, so as to improve libido, enhance erectile frequency and satisfaction, and increase bone mineral density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
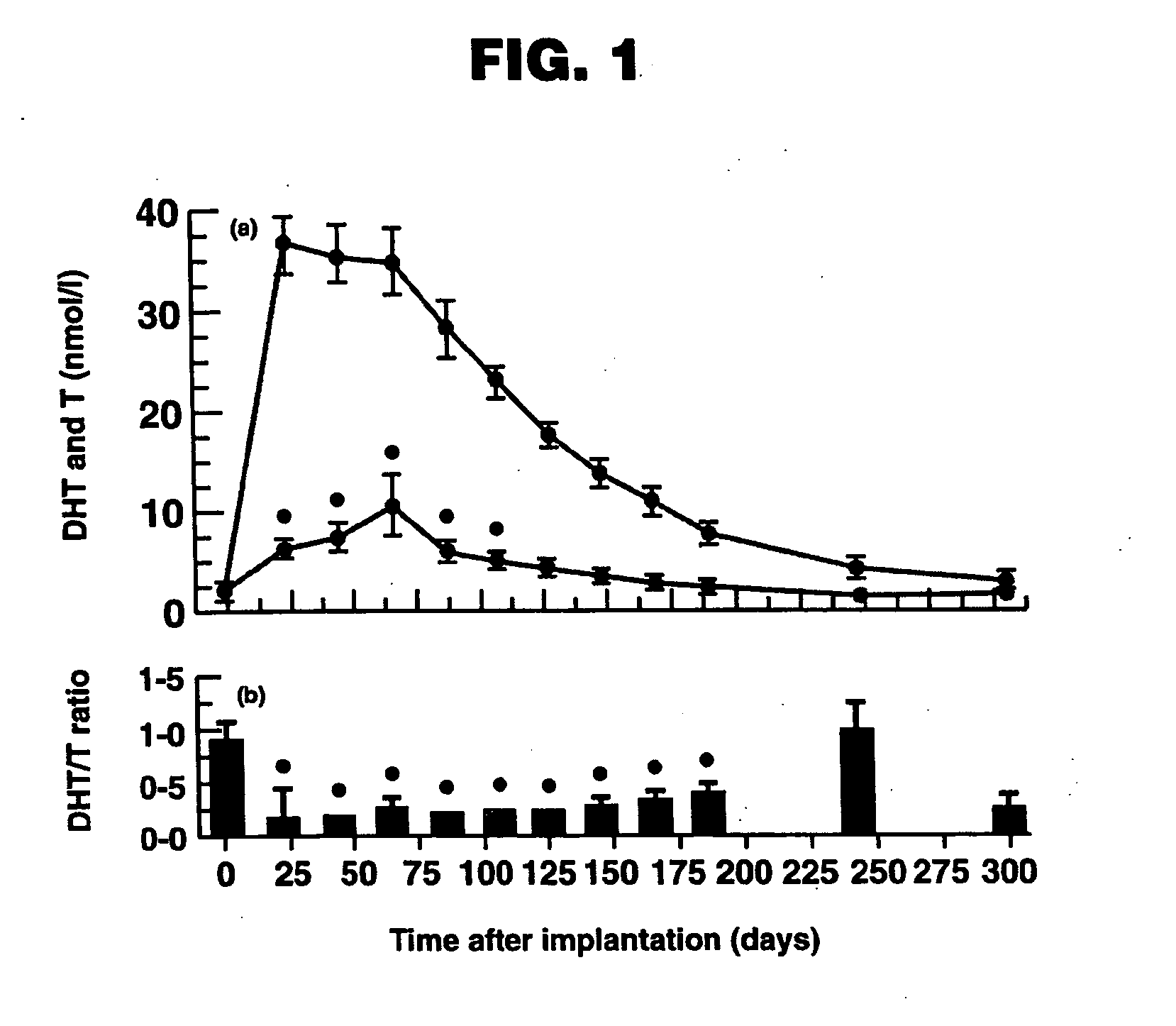
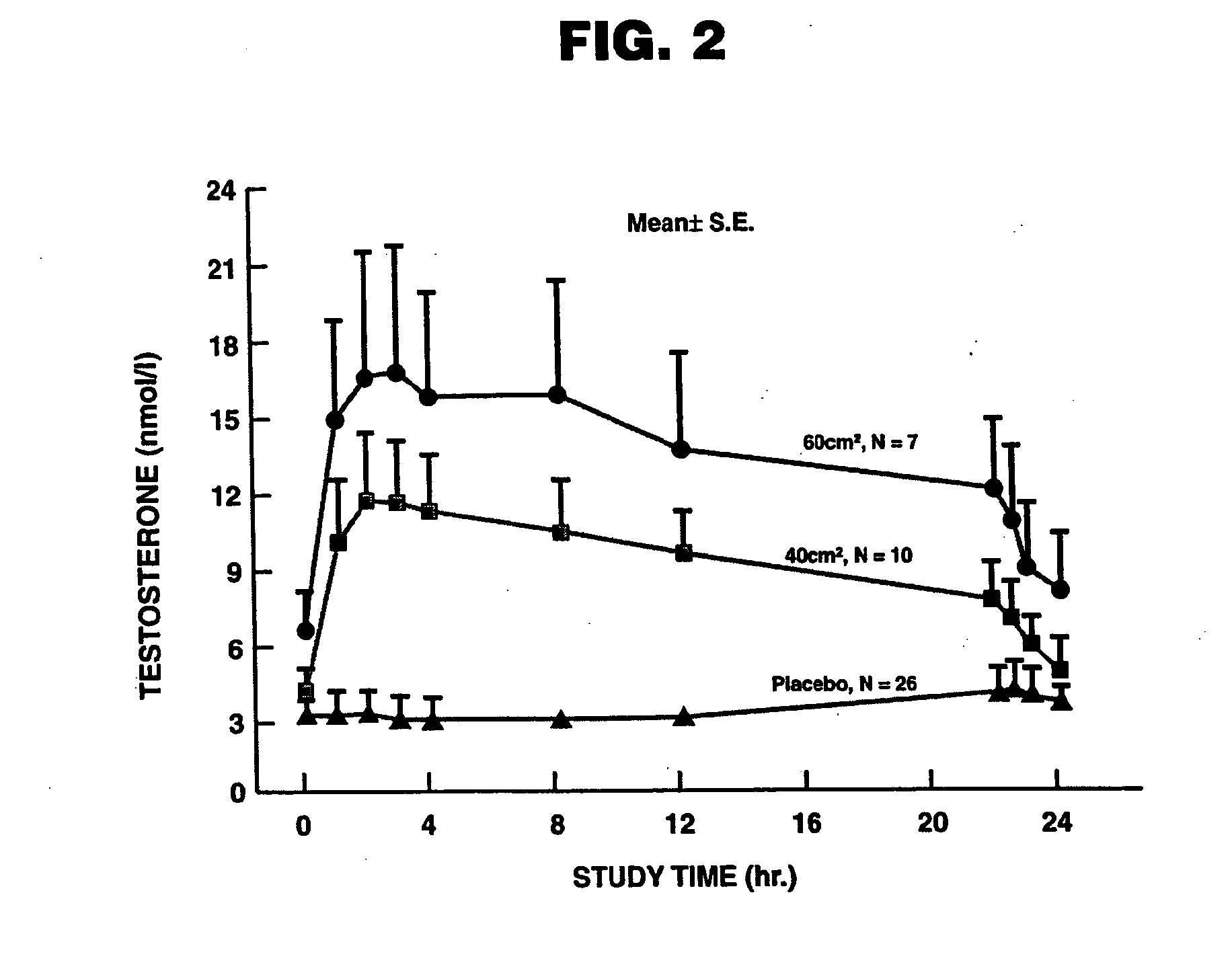
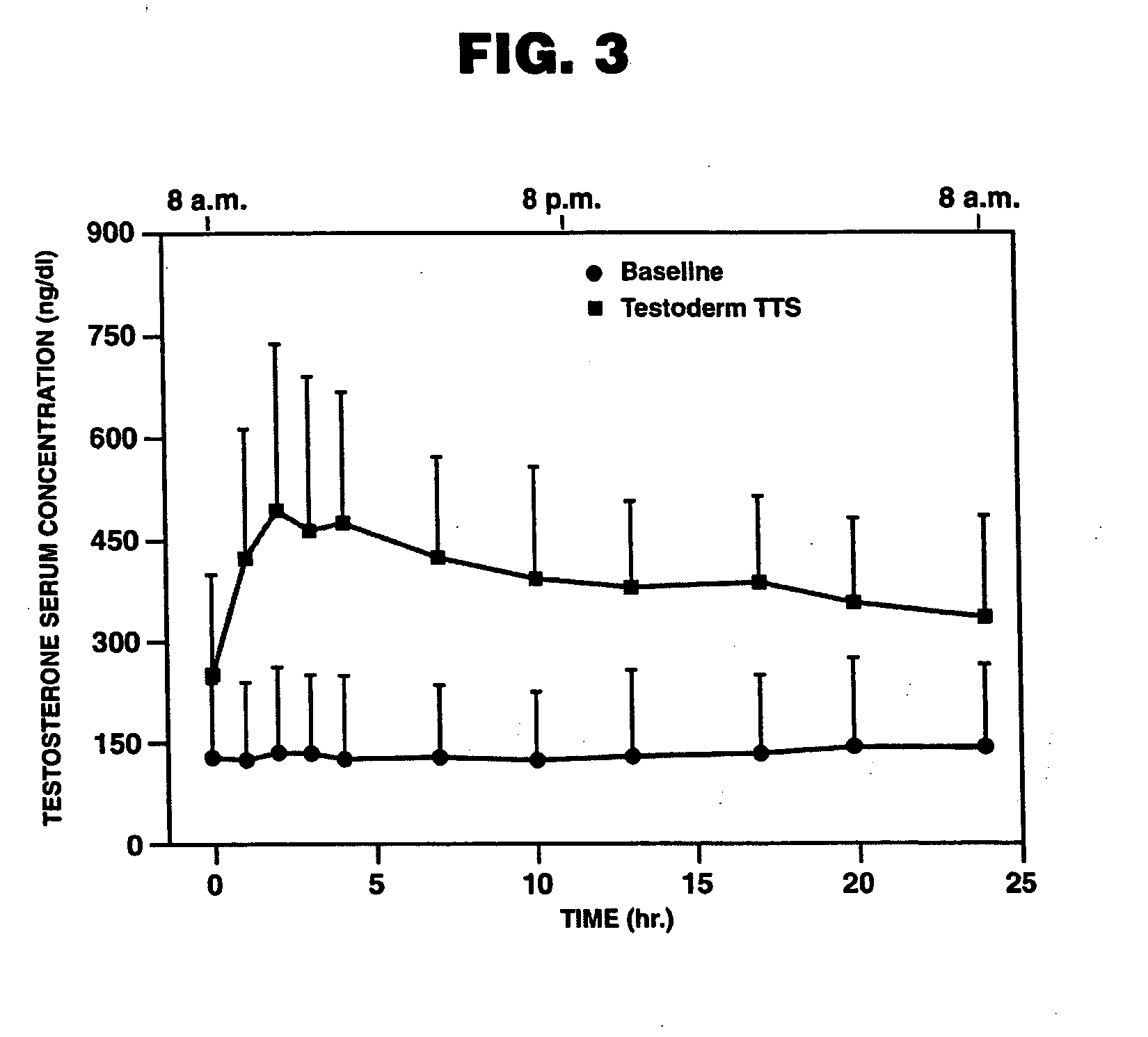
[0114]Treatment of Hypogonadism in Male Subjects. One embodiment of the present invention involves the transdermal application of ANDROGEL® as a method of treating male hypogonadism. As demonstrated below, application of the gel results in a unique pharmacokinetic profile for testosterone, as well as concomitant modulation of several other sex hormones. Application of the testosterone gel to hypogonadal male subjects also results in (1) increased bone mineral density, (2) enhanced libido, (3) enhanced erectile capability and satisfaction, (4) increased positive mood, (5) increased muscle strength, and (6) better body composition, such increased total body lean mass and decreased total body fat mass. Moreover, the gel is not generally associated with significant skin irritation.
[0115]Methods. In this example, hypogonadal men were recruited and studied in 16 centers in the United States. The patients were between 19 and 68 years and had single morning serum testosterone levels at scre...
example 2
[0240]Gel Delivery Dosage Forms and Devices. The present invention is also directed to a method for dispensing and packaging the gel. In one embodiment, the invention comprises a hand-held pump capable of delivering about 2.5 g of testosterone gel with each actuation. In another embodiment, the gel is packaged in foil packets comprising a polyethylene liner. Each packet holds about 2.5 g of testosterone gel. The patient simply tears the packet along a perforated edge to remove the gel. However, because isopropyl myristate binds to the polyethylene liner, additional isopropyl myristate is added to the gel in order to obtain a pharmaceutically effective gel when using this delivery embodiment. Specifically, when dispensing the gel via the foil packet, about 41% more isopropyl myristate is used in the gel composition (i.e., about 0.705 g instead of about 0.5 g in Table 5), to compensate for this phenomenon.
[0241]The composition can also be dispensed from a rigid multi-dose container (e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com