Process for preparation of calcium thiosulfate liquid solution from lime, sulfur, and sulfur dioxide
a technology of calcium thiosulfate and liquid solution, which is applied in the field of process for the preparation of calcium thiosulfate, a plant nutrient solution, can solve the problems of not being able to remove all ammonia, lingering ammonia odor and/or contamination of calcium thiosulfate, and sulfur dioxide being toxic to vegetation, etc., to achieve high purity level, reduce undesirable formation, and reduce the effect of ammonia concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
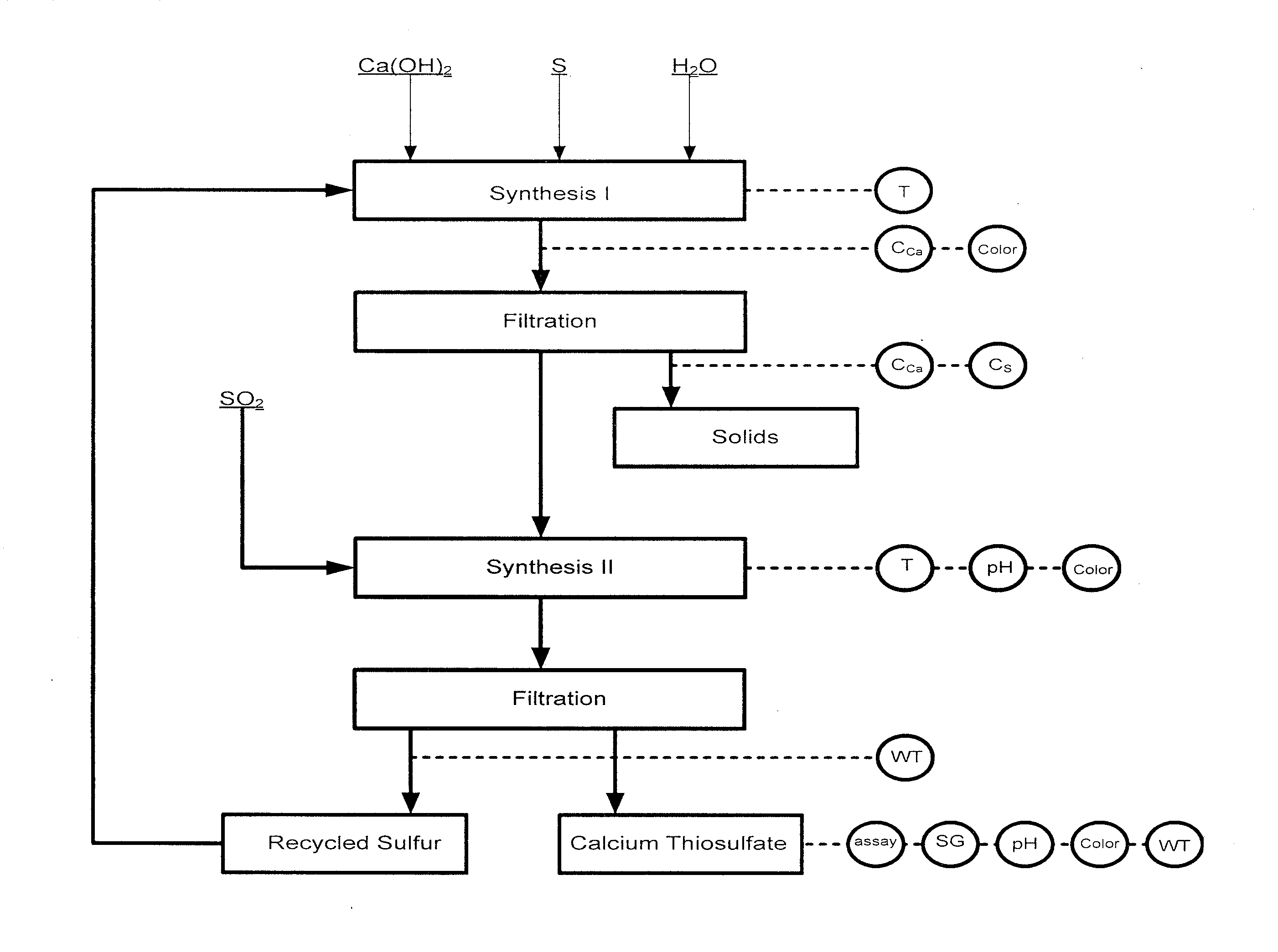
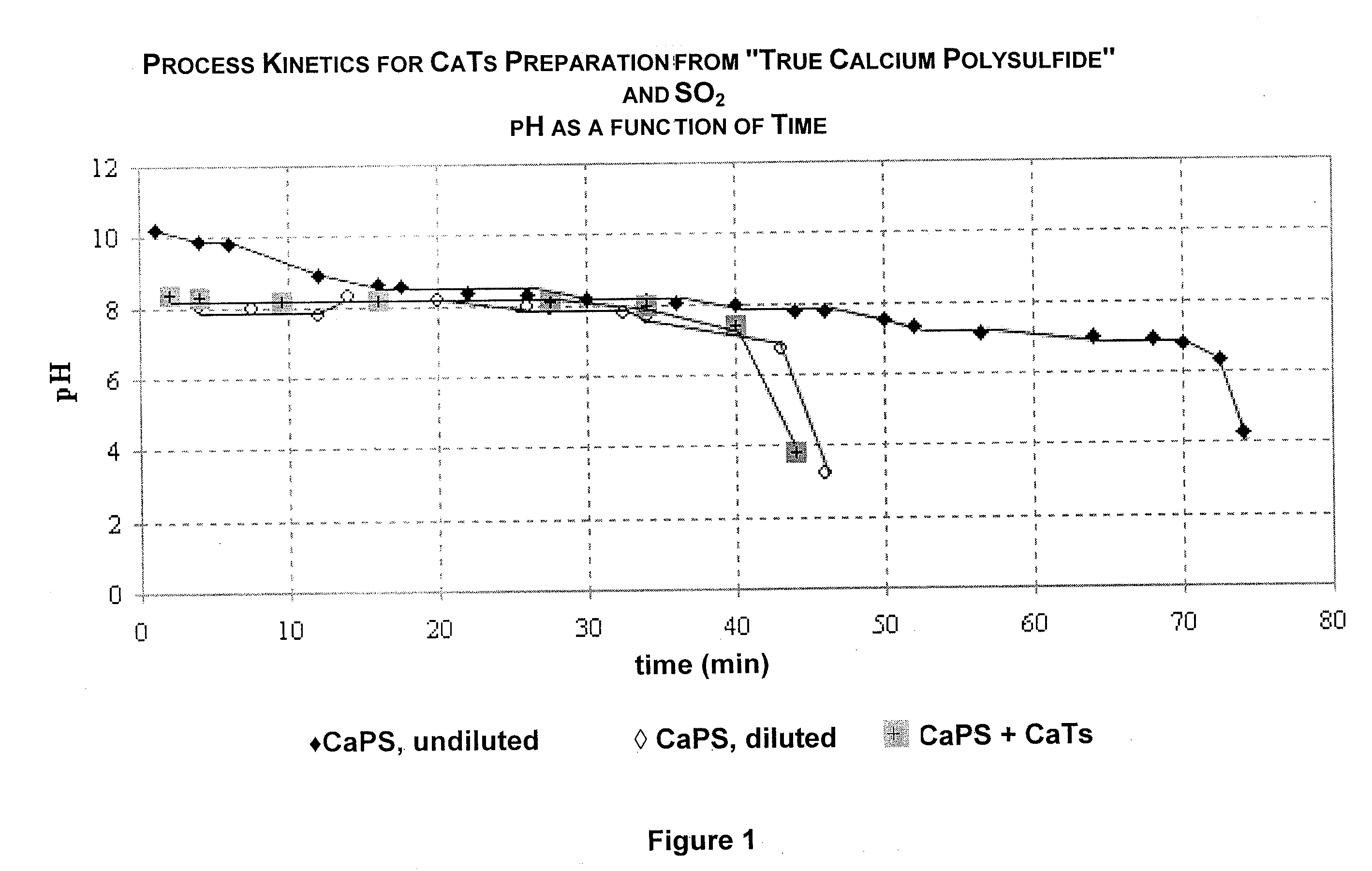
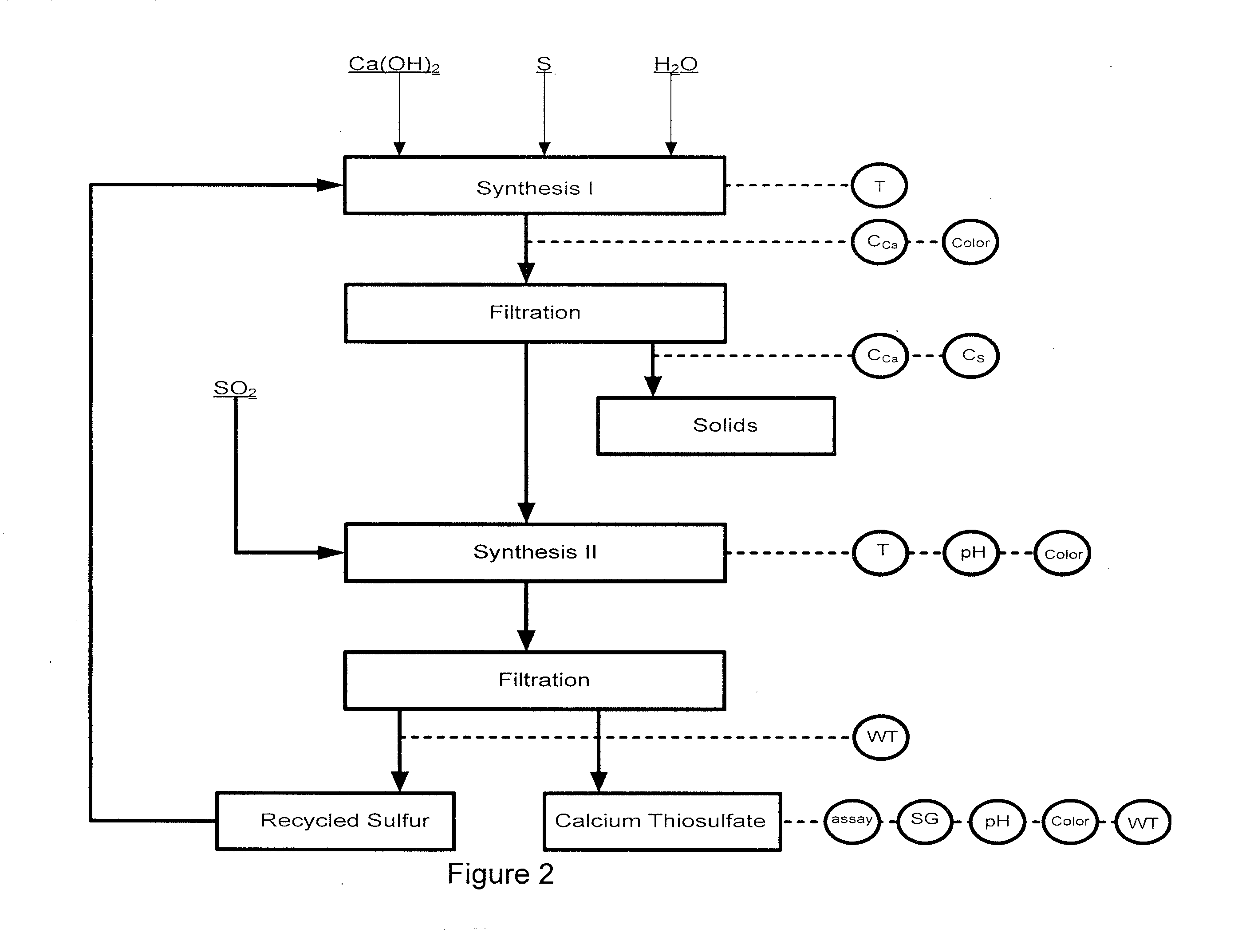
Image
Examples
experiment # 3
[0116]Experiment #3 was not completed due to the low recovery of calcium in step (i).
[0117]The inventors determined that the higher the CCa was in the lime-sulfur parent solution, the higher the CCa was in the calcium thiosulfate solution. This was true if CCaTs was less than about 27 wt %. The further increase of CCa in the lime-sulfur solution did not influence CCaTs, but resulted in the growth of the amount of solid.
[0118]The preferred temperature during the step (i) in which lime-sulfur is produced by reacting calcium hydroxide and sulfur is about 93° C. to about 99° C. (200° F.-210° F.) as described in U.S. Pat. No. 6,984,368.
[0119]The inventors determined that the duration of the step (i) appears to be dependent on molar ratio only (since temperature is not variable).
[0120]While using pure lime (99% or better) and pure sulfur with the optimal molar ratio of S:Ca(OH)2:H2O=(3.6-3.8):1:25.5, the endpoint of step (i) could be judged by the appearance of the solution. The endpoint ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com