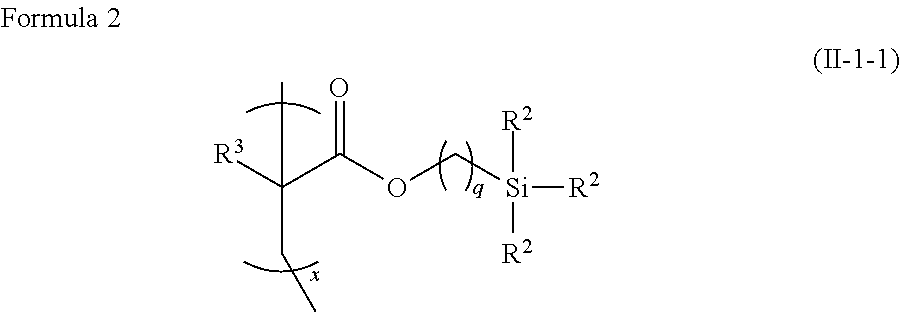
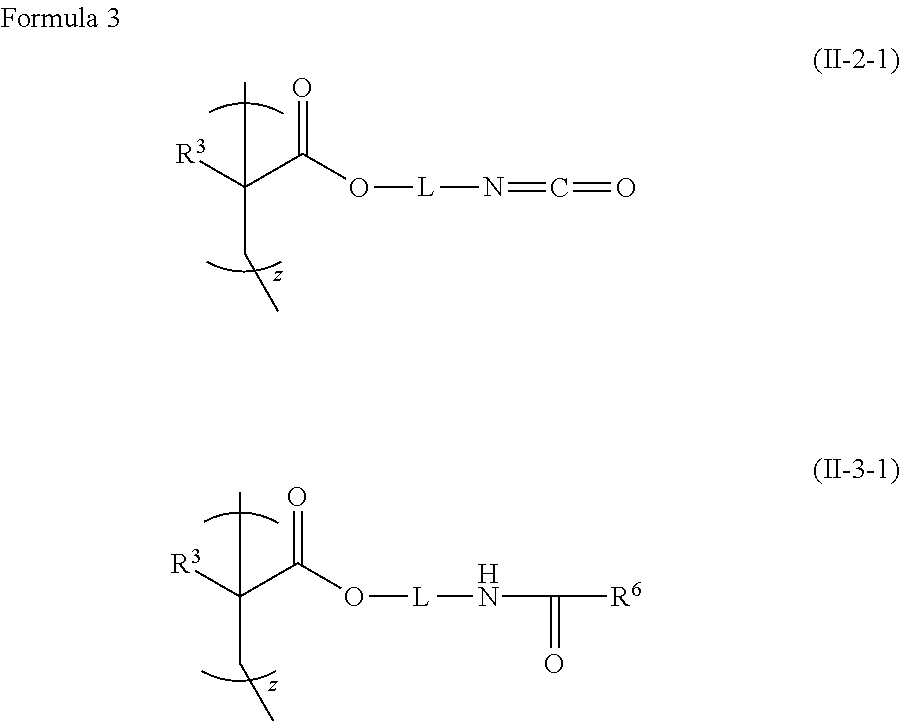
Polymer composition having photoalignable group, liquid crystal alignment film formed of the polymer composition, and optical device having phase difference plate formed of the liquid crystal alignment film
a polymer composition and photoaligning technology, which is applied in the direction of instruments, polarising elements, optical elements, etc., can solve the problems of dust or static electricity generation, luminance inversion, dust and static electricity generation, etc., and achieve high optical uniformity, excellent sensitivity, and high alignment stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
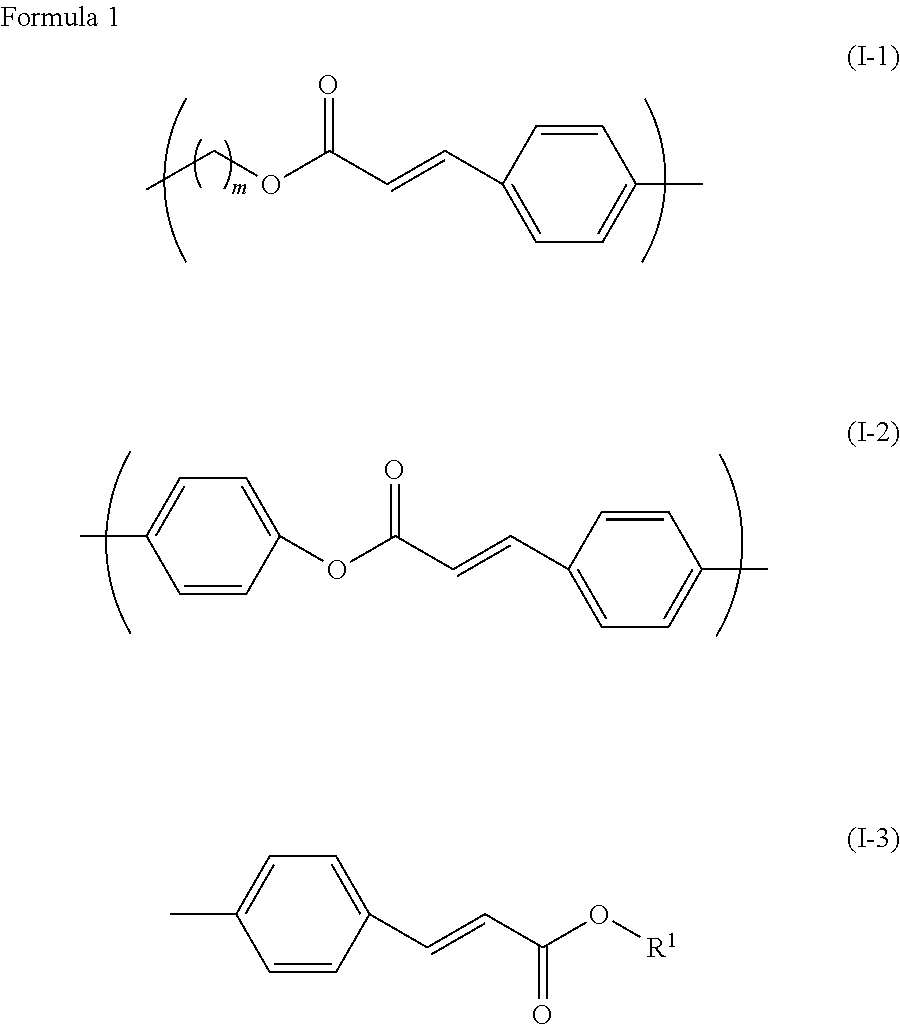
[0176]Monomer (1-1) having a photoalignable group (as included in monomer I-1-1-a described above) was prepared as described below.
[0177]Then, 22.8 g of methoxycinnamic acid, 16.7 g of 2-hydroxybutyl methacrylate and 3.2 g of 4-dimethylaminopyridine (DMAP) were added to 230 mL of dichloromethane, and the resultant mixture was stirred under a nitrogen atmosphere. Thereto, a dichloromethane (60 mL) solution containing 27.7 g of 1,3-dicyclohexylcarbodiimide (DCC) was added dropwise. After dropwise addition, the resultant mixture was stirred at room temperature for 8 hours. A precipitate formed was filtered off, and an organic layer was washed with water and dried over anhydrous magnesium sulfate. A solvent was distilled off under reduced pressure, a residue was purified by means of column chromatography, and recrystallized in ethanol, and thus 30.0 g of monomer (1-1) having the photoalignable group was obtained. Measurement results of phase transition temperature and nuclear magnetic r...
synthesis example 2
[0180]Monomer (1-2) having a photoalignable group (as included in monomer I-2-1-a described above) was prepared as described below.
(First Step)
[0181]Then, 1.120 mmol of ethyl 4-hydroxycinnamate, 1.230 mmol of sodium hydroxide and 120 mmol of sodium iodide were added to 1,000 mL of N,N-dimethylformamide (DMF), and the resultant mixture was stirred at 60° C. under a nitrogen atmosphere. Thereto, 1.230 mmol of 6-chlorohexanol was added dropwise. After dropwise addition, the resultant mixture was stirred at 80° C. for 8 hours. Ethyl acetate and water were added to a reaction mixture, and an organic layer was extracted. The resultant organic layer was washed with water and dried over anhydrous magnesium sulfate. A solvent was distilled off under reduced pressure. The resultant residue and 1.230 mmol of sodium hydroxide were added to a mixed solution of water (800 mL) and methanol (800 mL), and the resultant mixture was stirred for 3 hours under heating reflux. A solvent was distilled off...
synthesis example 3
[0186]Monomer (1-3) having a photoalignable group (as included in monomer I-3-1-a described above) was prepared as described below.
(First Step)
[0187]Then, 50 g of 2-hydroxybutyl methacrylate and 50 mL of pyridine were added to 150 mL of toluene, 80 g of p-toluenesulfonic acid chloride was added under cooling, and the resultant mixture was stirred at room temperature for 16 hours under a nitrogen atmosphere. A precipitated salt was removed by filtration under reduced pressure. Water (100 mL) was added to a filtrate, and the resultant mixture was stirred at 40° C. for 2 hours. An organic layer was separated, and the resultant organic layer was sequentially washed with 2 N hydrochloric acid, a saturated aqueous solution of sodium hydrogencarbonate, and water, and dried over anhydrous magnesium sulfate. Toluene was distilled off under reduced pressure, and thus 98 g of crude colorless liquid (ex-3) was obtained.
(Final Step)
[0188]Then, 30 g of compound (ex-4) and 7.4 g of sodium hydroxid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com