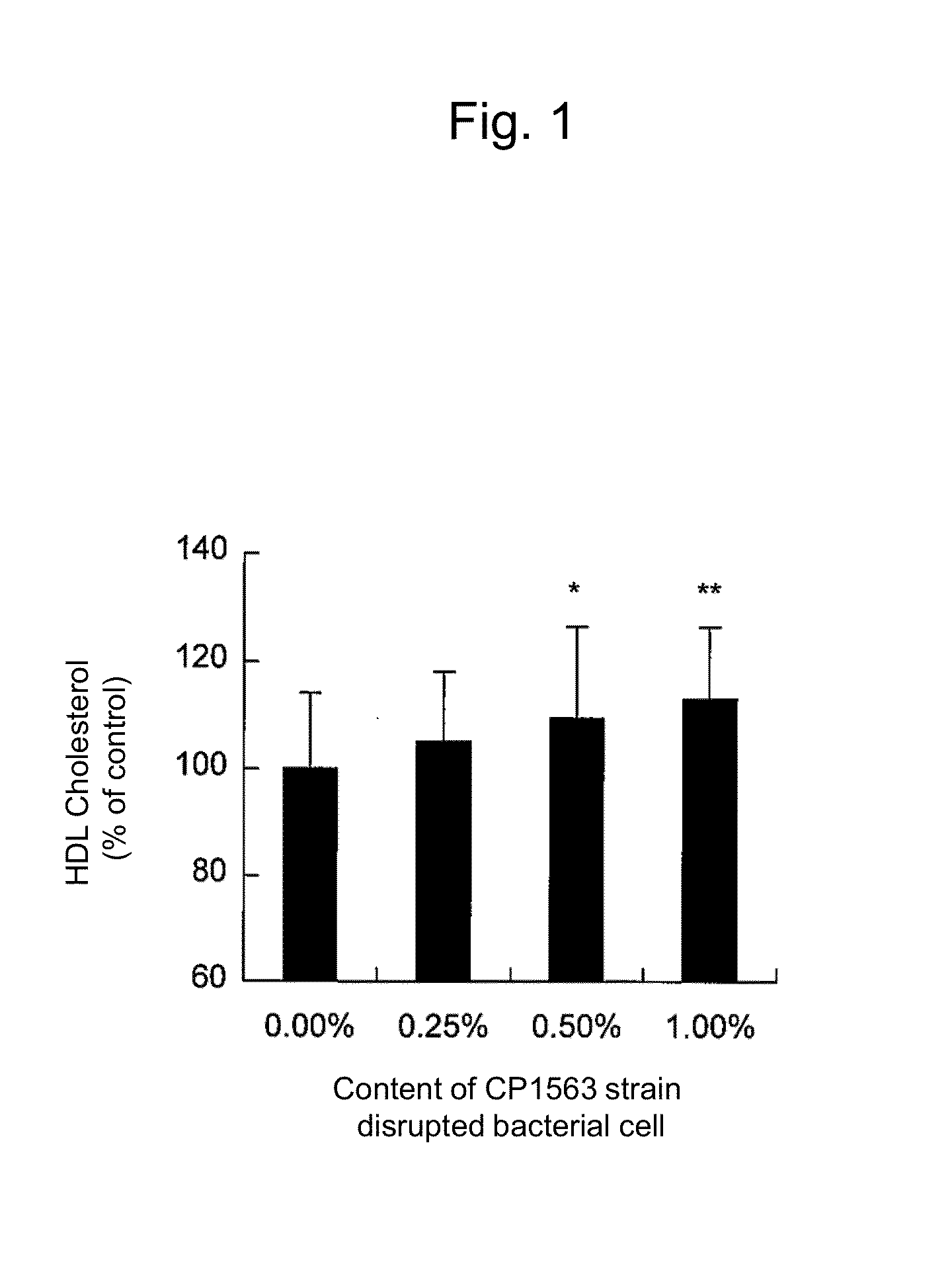
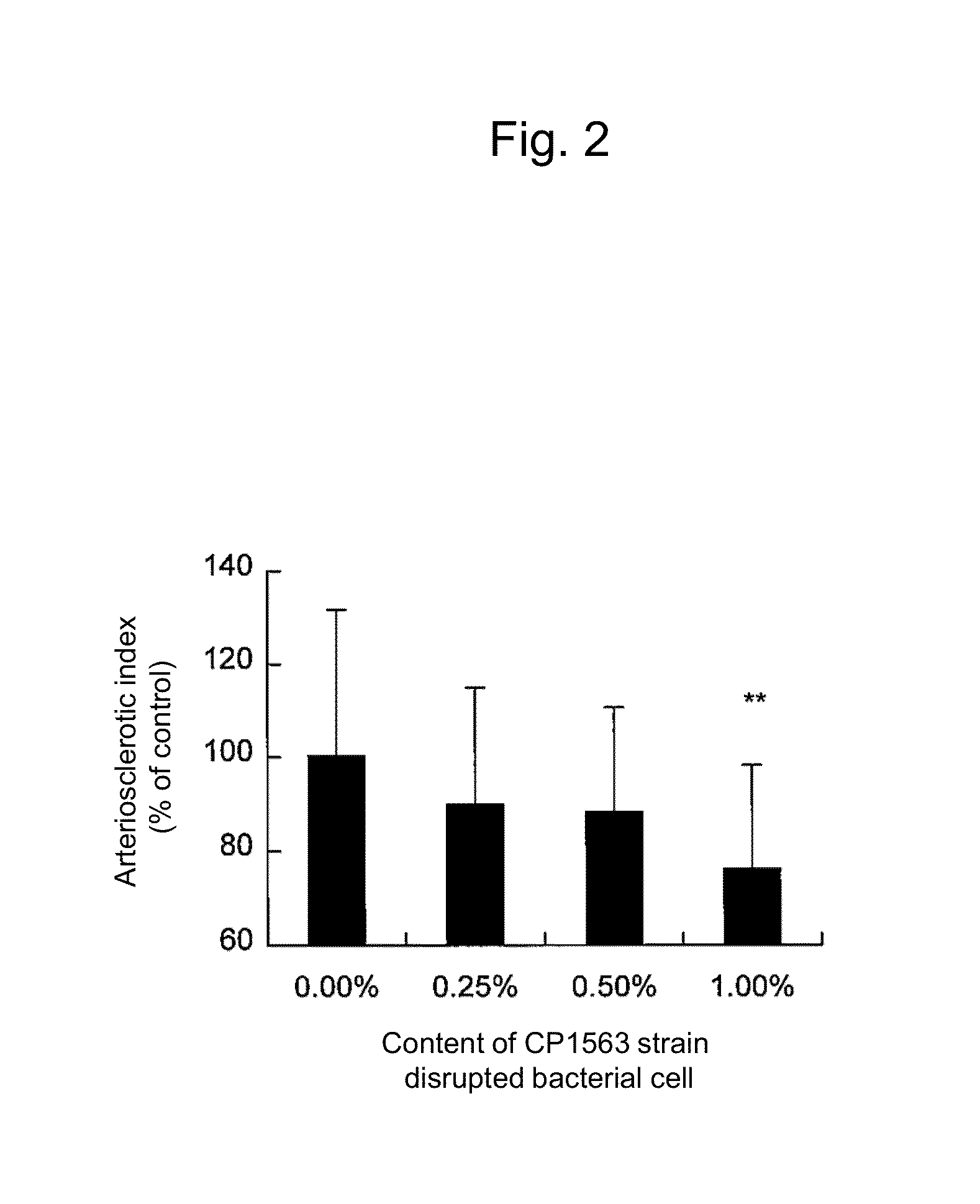
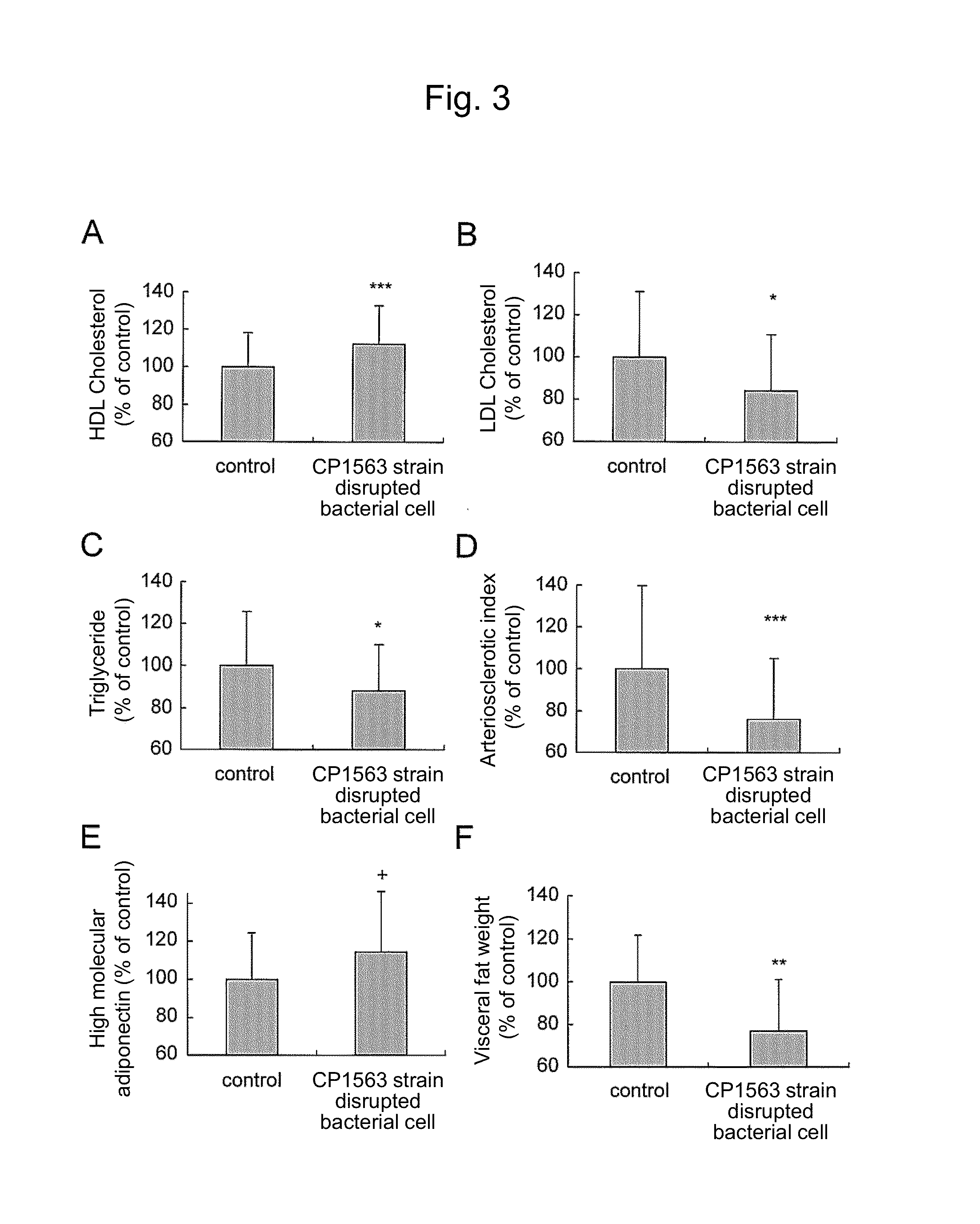
Lipid metabolism and/or sugar metabolism improver containing lactic acid bacterium or treatment product thereof
a technology of lactic acid bacteria and lipid metabolism, which is applied in the direction of bacteria material medical ingredients, metabolic disorders, drug compositions, etc., can solve the problems of insufficient effect, adverse effects of ppars, and inability to activate ppars or other satisfactory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Bacterial Cell Powder
[0111]The cell strain of each lactic acid bacterium was inoculated from frozen stocks onto plate medium, and then the pre-preincubation, preincubation and main incubation were carried out (5 ml→40 ml→2 L) using liquid medium. Table 1 shows the bacterial species, bacterial strains, medium and incubation temperatures used. Additionally, the inoculum concentration was 1% of each liquid medium weight and the incubation was carried out for 18 hours (see Table 2 for the medium used and incubation temperatures). After the incubation, the culture was centrifuged at 12000 g at 10° C. for 7 minutes, and the supernatant was removed. Ion exchange water was added, the culture was centrifuged in the same manner, and the freeze-dried bacterial cell was dispersed using a mill (TESCOM), thereby obtaining a bacterial cell powder.
TABLE 1BacterialCulturePlateLiquidBacterial species namestrain No.temperaturemediummediumEnterococcus faecalis137MRSMRSLactobacillus gallin...
example 2
PPARα Reporter Assay
[0114]Cultured cell CV-1 derived from the kidney of an African green monkey was prepared to a concentration of 5×104 cells / ml, suspended in DMEM medium containing 10% (v / v) FBS (SIGMA), and cultured at 37° C. for 24 hours under 5% CO2 in air (v / v) in a concentration of 500 μL / well using a flat 24-well plate (Corning). Twenty four hours later, 80 to 90% confluence was microscopically confirmed to have reached, and subsequently the transfection was carried out by the following procedure.
[0115]Plasmid pM-PPARα 0.16 which comprises a DNA fragment encoding a chimeric protein comprising PPARα ligand binding domain (derived from human) and GAL-4DNA binding domain (derived from yeast), and p4xUASg-tk-luc 0.16 μg, which is a luc (derived from sea-firefly) reporter gene plasmid designed to receive expression control by the above chimeric protein, and pRL-CMV 0.016 μg, which is a luc (derived from Renilla) expression plasmid having a viral expression promoter with a fixed e...
example 3
PPARγ Reporter Assay
[0122]Cultured cell CV-1 derived from the kidney of an African green monkey was prepared to a concentration of 5×104 cells / ml, suspended in DMEM medium containing 10% (v / v) FRS (SIGMA), and cultured at 37° C. for 24 hours under 5% CO2 in air (v / v) in a concentration of 500 μL / well using a flat 24-well plate (Corning). Twenty four hours later, 80 to 90% confluence was microscopically confirmed to have reached and subsequently the transfection was carried out by the following procedure.
[0123]Plasmid pM-PPARα 0.16 μg, which comprises a DNA fragment encoding a chimeric protein comprising PPARγ ligand binding domain (derived from human) and GAL-4DNA binding domain (derived from yeast), and p4xUASg-tk-luc 0.16 μg, which is a luc (derived from sea-firefly) reporter gene plasmid designed to receive expression control by the above chimeric protein, and pRL-CMV 0.016 μg, which is a luc (derived from Renilla) expression plasmid having a viral expression promoter with a fixe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com