Methods and compositions to treat myocardial conditions
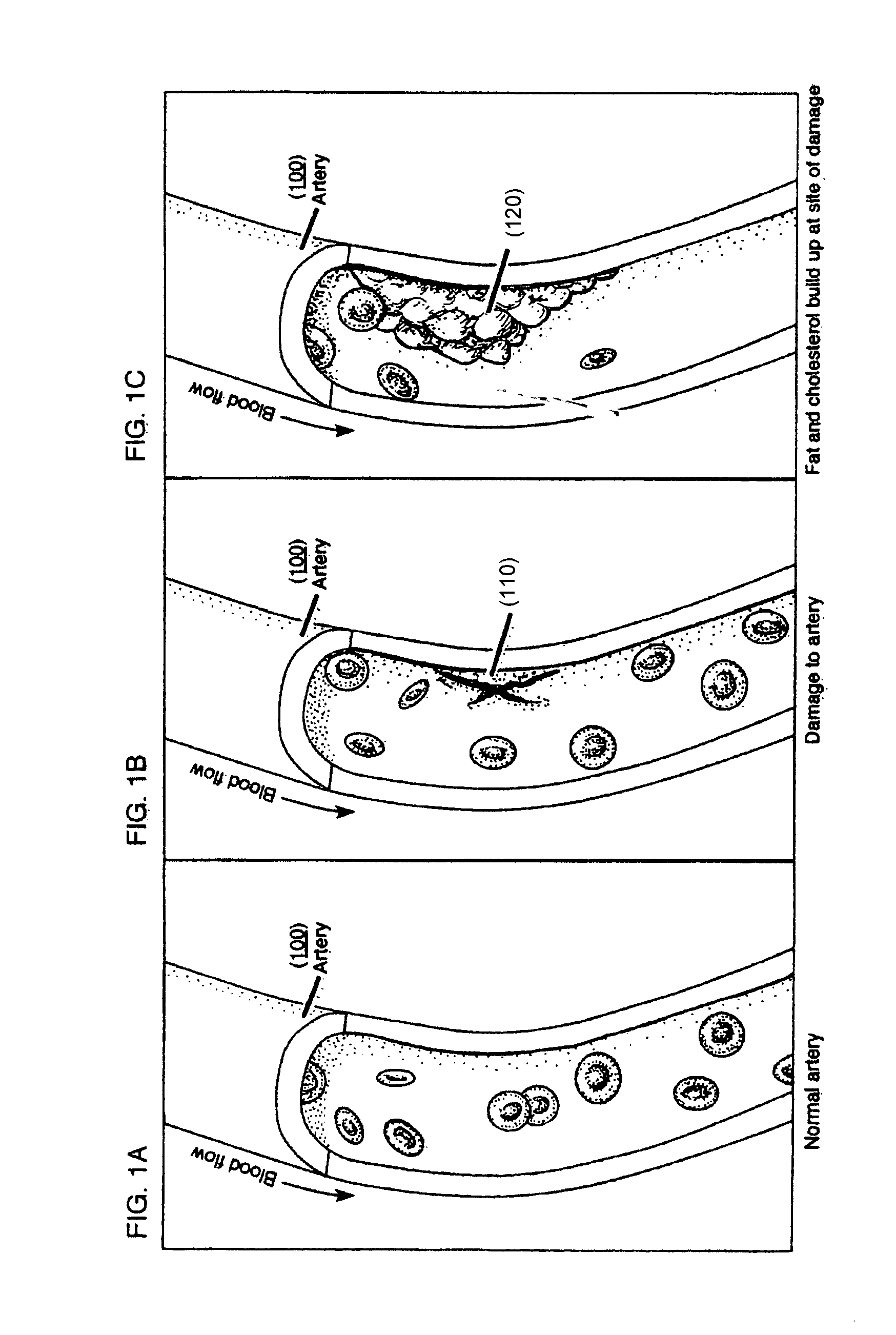
a myocardial infarction and composition technology, applied in the field of myocardial infarction treatment, can solve the problems of sudden death in adults, blockage of blood flow, and imbalance between myocardial blood flow and metabolic demand of the myocardium, so as to prevent remodeling, reduce mechanical stress, and strengthen the infarct region
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
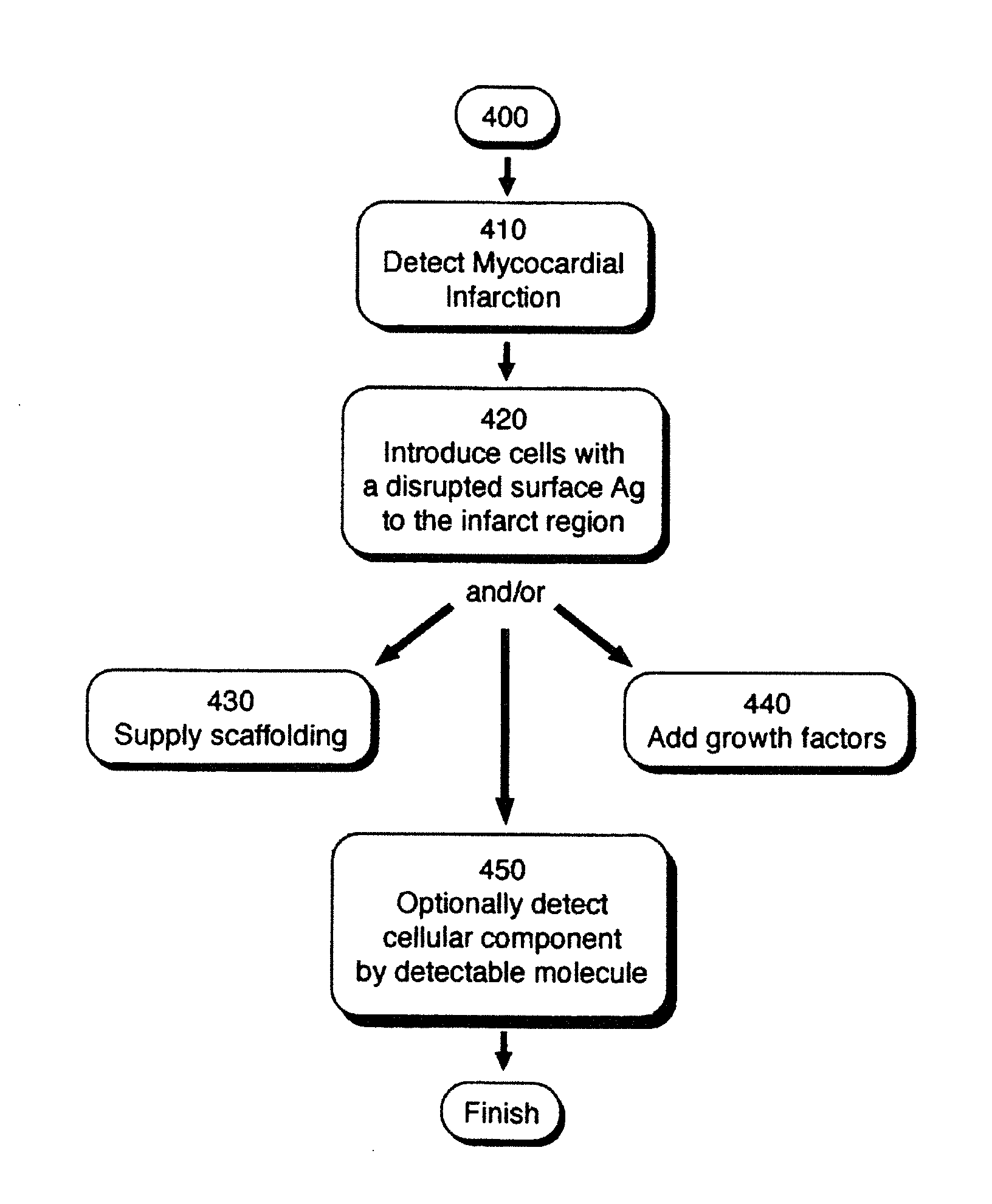
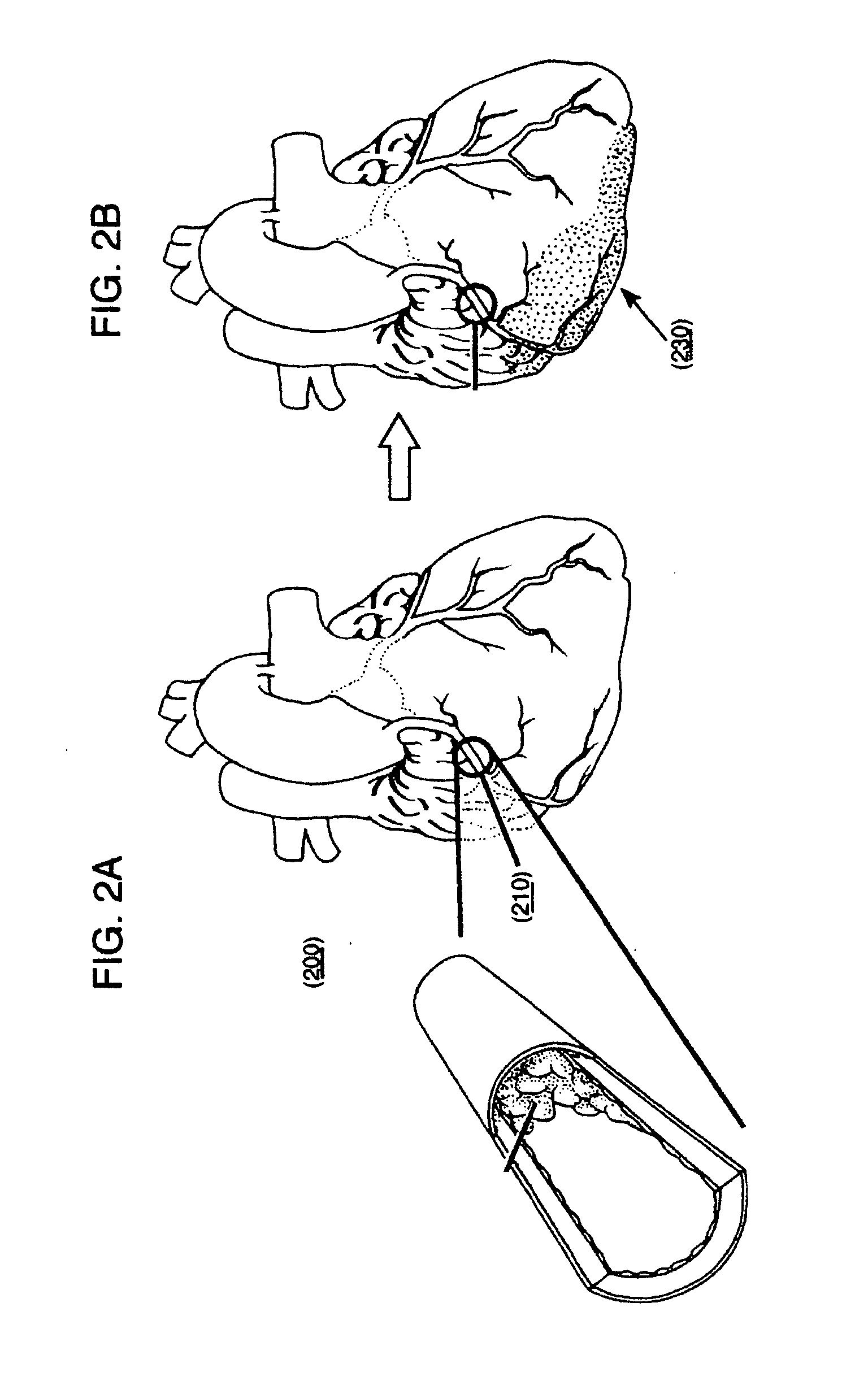
[0213]In one example a 2-component gel may be injected via a dual needle catheter to an infarct region. One possible 2-component gel material may include Na-Alginate (component 1) which will likely ionically crosslink (often within seconds) when added to a soluble solution of calcium, barium and / or strontium (component 2). One potential crosslinker for the components may be CaCl2 (calcium chloride). In one example, covalently conjugating a peptide or protein to some of the acid groups of the alginate may enhance a cellular response. For example, conjugation of RGD groups or gelatin may promote cell adhesion, since these are binding sites for cells. The amine (N) terminus of gelatin or RGD may be conjugated to the acid groups of alginate via carbodiimide chemistry, forming an amide. The reaction may be mediated to higher yield with fewer side products (such as the inactive N-acyl urea) by first forming an active ester with, for example, 1-hydroxybenzotriazole or N-hydroxy succinimide...
example 2
[0215]In one example, a person with an mild or severe desynchrony (such as left bundle branch block), detectable through QRS duration, echocardiography, or other means, and with a history of a previous myocardial infarction, may first receive a catheter delivered injection of micronized porcine urinary bladder matrix (UBM) particulate (cryogenically ground UBM and resuspended at 5% weight / volume in PBS) to achieve alterations in the mechanical properties of the previous / scarred region. Following injection(s), implantation of a cardiac rhythm management device may be used to provide cardiac resynchronization therapy (CRT) to alter the desynchronized condition and alter the patient's progression to heart failure.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com