Methods For Producing Metal Powders And Metal Masterbatches
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1 (
Theoretical Example)
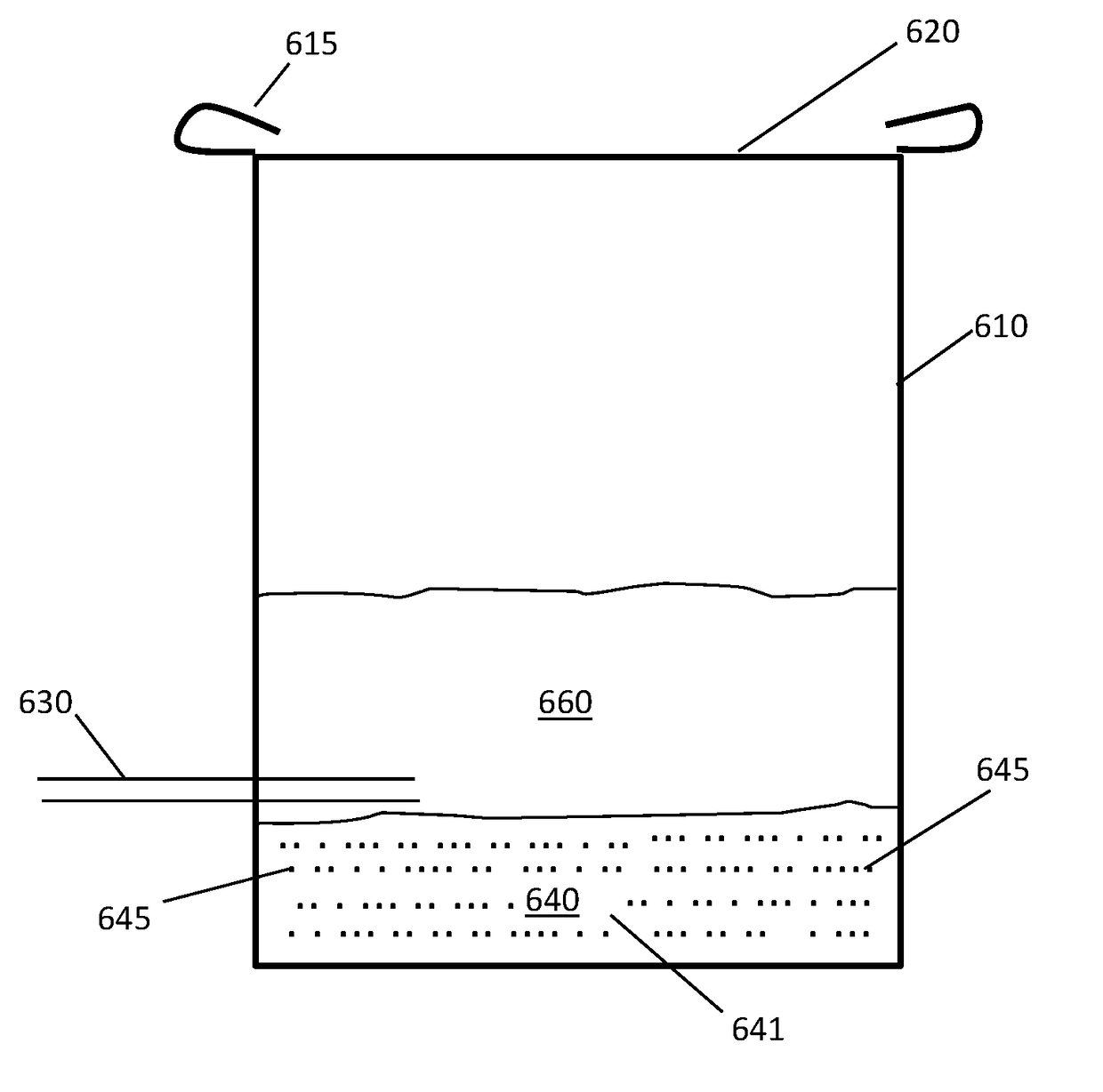
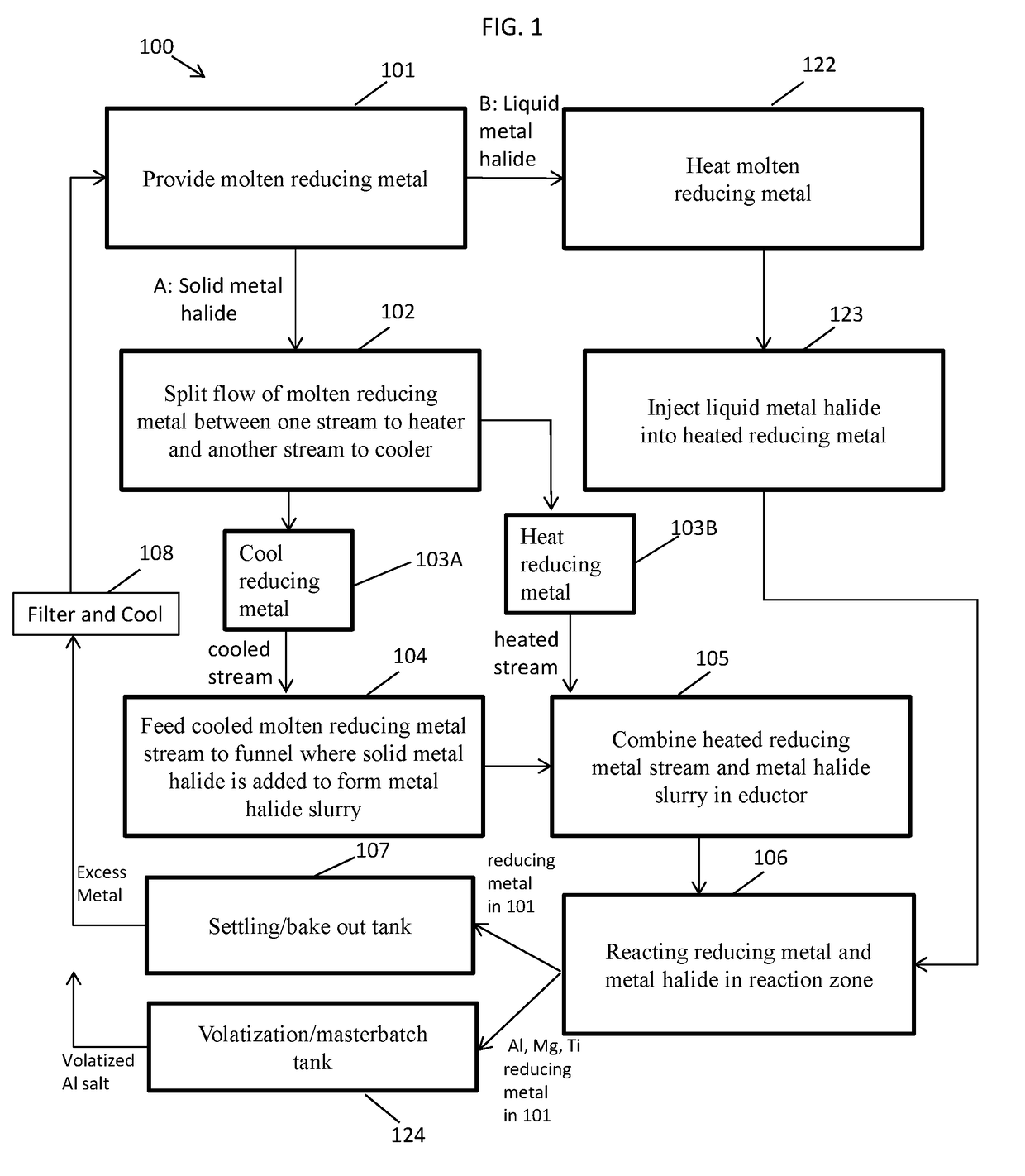
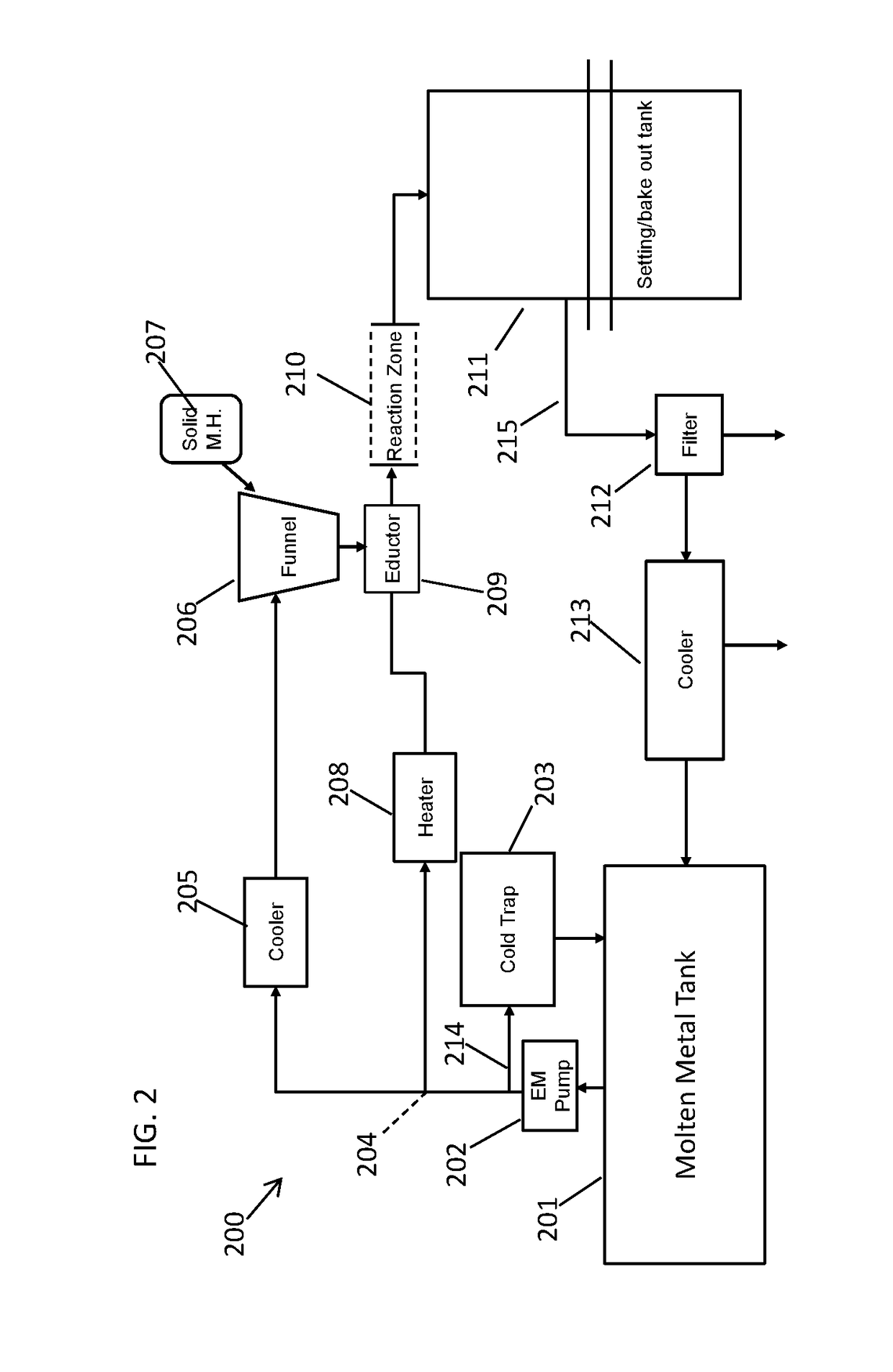
[0071]Using a process flow as illustrated in FIG. 2, a storage tank containing 200 gallons of molten sodium is pumped through a continuous loop using an electromagnetic pump. There is a cold trap installed on the loop that is used to remove contaminants prior to starting up production. Once the contaminants are removed the cold trap is valved off until needed again. The electromagnetic pump pumps the molten sodium to a flow split. The flow to the cooler can be roughly 3 GPM and the flow to the heater can be roughly 17 GPM. The flows can be controlled using valves and Coriolis flow meters.
[0072]The cold sodium stream flows around a funnel. The HfCl4 powder is added to this funnel. The sodium flows around the funnel and collects the powder and it is drawn through an eductor. The eductor uses the hot sodium flow as the motive fluid sucking the HfCl4 slurry into it. The additional heat provided by the hot sodium stream initiates the reduction reaction. Feeding of the...
example 2
wder Feed Test
Instrumentation Setup
[0075]A powder trickier was used for all halide powder trials to feed the reactant powders to a beaker containing alkali metal(s). This powder feeder consists of an adjustable hopper, discharge tube, stand, and 2-speed control pad. All reactant powders flowed readily through the tube given the vibration frequency at hand, except the TaCl5 and NiCl2 50 / 50 powder blend. This powder blend packed tightly inside both the tube and the hopper base. As a result, remaining powder was fed to the reaction beaker using a “hand-add” approach with a spatula for the TaCl5 and NiCl2 50 / 50 blend.
[0076]All tests utilized an IKA 70 Watt mixer with the capability of producing speeds from 60 to 2000 rpm. A stainless steel, 1.20 inch diameter, turbine impeller blade was utilized for the first two tests performed, TaCl5 in excess sodium. All subsequent tests were performed using a stainless steel, 1.65 inch diameter, Cowles blade impeller to improve the incorporation of ...
example 3
wder and Liquid Initiation Test
Instrumentation Setup
[0101]A second set of experiments was conducted to verify the reactivity of various powder and liquid halides with sodium metal. All tests were performed in a glovebox, inerted with argon to eliminate oxygen and moisture from the atmosphere.
[0102]Powder halide transfer: Aluminum Trichloride and Zirconium (IV) Chloride powders were transferred into weighing dishes using a microspatula. The powders were then poured into the reaction cups from the weighing dishes.
[0103]Liquid halide transfer: Vanadium (IV) Chloride, Tin (IV) Chloride, Titanium (IV) Chloride, and Silicon Tetrachloride were transferred into the reaction cups using 1 mL syringes. For each liquid halide, a volume of 0.1 mL was transferred into a syringe. The syringes were then placed in the glovebox. The syringes were then used to inject drops of each liquid halide into a reaction cup containing molten sodium metal.
[0104]Reaction vessel: Stainless steel 2.5 oz. cups were ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com