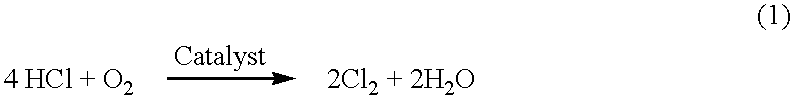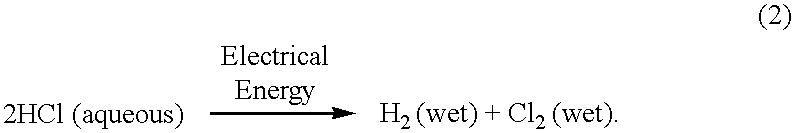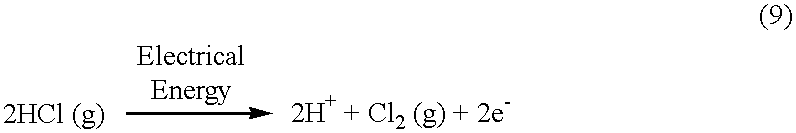Electrochemical conversion of anhydrous hydrogen halide to halogen gas using a cation-transporting membrane
an anhydrous hydrogen halide and cation-transporting technology, which is applied in the direction of fuel cell details, final product manufacture, climate sustainability, etc., can solve the problems of environmental pollution, acid or chloride ions discharged into waste water streams, and the inability to sell or use hydrogen chloride or the acid produced, etc., to achieve the effect of reducing investment costs, and reducing overall cell voltag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
The electrochemical cell of the first embodiment also comprises a structural support for holding the cell together. Preferably, the support comprises a pair of backing plates which are torqued to high pressures to reduce the contact resistances between the current collectors and the electrodes. The plates may be aluminum, but are preferably a corrosion-resistant metal alloy. The plates include heating elements (not shown) which are used to control the temperature of the cell. A non-conducting element, such as TEFLON.RTM. or other insulator, is disposed between the collectors and the backing plates.
The electrochemical cell of the first embodiment also includes a voltage source (not shown) for supplying a voltage to the cell. The voltage source is attached to the cell through current collectors 30 and 32 as indicated by the + and - terminals, respectively, as shown in FIG. 1.
When more than one anode-cathode pair is used, such as in manufacturing, a bipolar arrangement is preferred. In...
second embodiment
FIG. 2 illustrates the present invention. Wherever possible, elements corresponding to the elements of the embodiment of FIG. 1 will be shown with the same reference numeral as in FIG. 1, but will be designated with a prime (').
In accordance with the second embodiment of the present invention, there is provided an electrochemical cell for the direct production of essentially dry halogen gas from anhydrous hydrogen halide. This cell will be described with respect to a preferred embodiment of the present invention, which directly produces essentially dry chlorine gas from anhydrous hydrogen chloride. However, this cell may alternatively be used to produce other halogen gases, such as bromine, fluorine and iodine from a respective anhydrous hydrogen halide, such as hydrogen bromide, hydrogen fluoride and hydrogen iodide. Such a cell is shown generally at 10' in FIG. 2. In this second embodiment, water, as well as chlorine gas, is produced by this cell.
Cell 10' comprises a cation-transp...
example 1
In this Example, a non-steady state electrochemical experiment (i.e., of a duration of five minutes for each potential setting) generating chlorine and hydrogen was performed in an electrochemical cell which was 1 cm..times.1 cm. in size. Platinum (Pt) extended with carbon was used for the cathode, and ruthenium oxide (RuO.sub.2) extended with carbon was utilized in the anode. The anode and cathode each contained 0.35 mg. / cm..sup.2 precious metal. The anode and the cathode were both bonded to the membrane, which was made of NAFION.RTM. 117. The potential from the power source was stepped in 0.10 volt increments from 1.0 to 2.0 volts. At each 0.10 volt increment, the potential was maintained for five minutes. The current response at the specific cell potentials was recorded at three different temperatures, namely 40.degree. C., 60.degree. C. and 80.degree. C., in order to assess the importance of this variable upon cell performance and is given in Table 1 below.
TABLE 1 Current Value ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| voltages | aaaaa | aaaaa |
| equivalent weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com