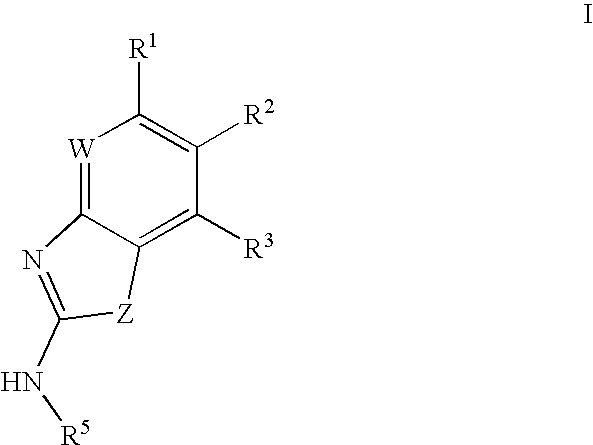
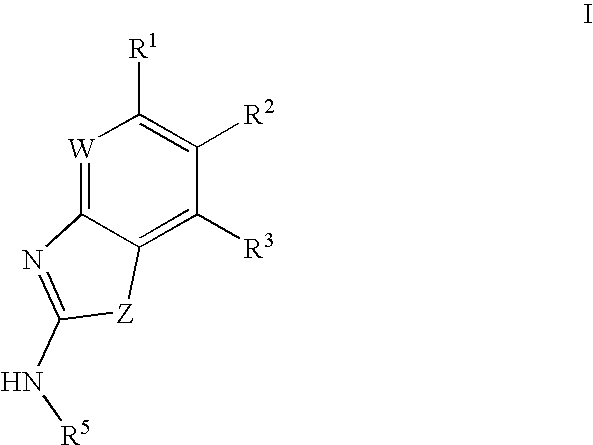
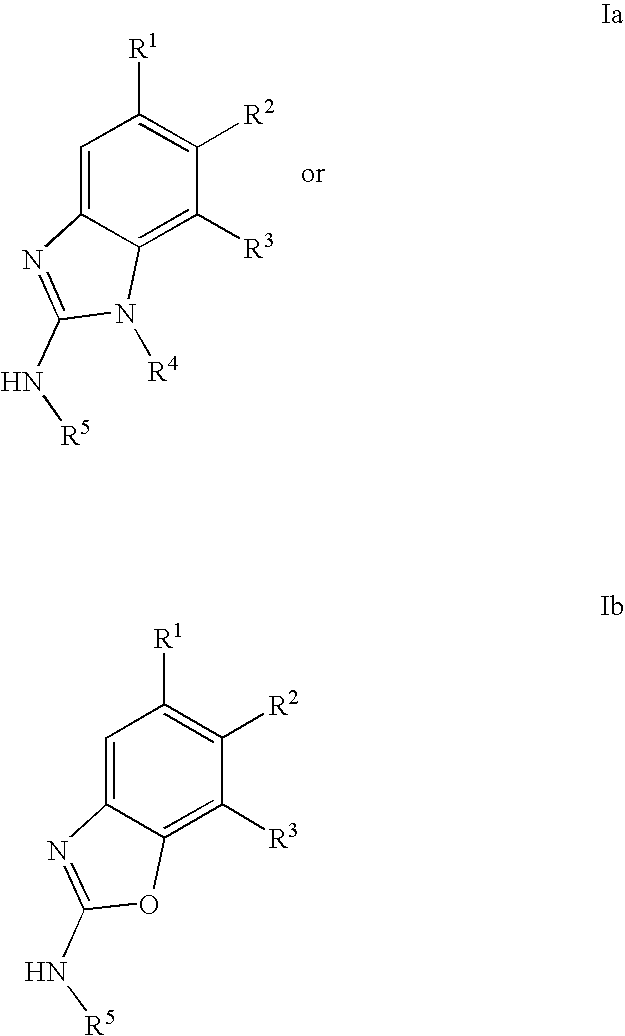
Gyrase inhibitors and uses thereof
a gyrase inhibitor and inhibitor technology, applied in the field of medicinal chemistry, can solve the problems of bacterial infections that are difficult to treat with antibiotics or even untreatable, and are considered to be a serious worldwide health problem, and the problem of bacterial infections is becoming particularly serious
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
4-(Pyridin-3-yl)-2-nitroaniline (2): To a solution of 4-bromo-2-nitroaniline (217 mg, 1 mmol) in DMF (6 mL) was added 3-pyridine boronic acid (148 mg, 1.2 mmol), potassium phosphate (276 mg, 1.3 mmol), and dichloro(diphenylphosphinoferrocene)palladium (75 mg, 0.1 mmol).
The resulting mixture was heated at 95° C. for 18 hours then cooled to room temperature and diluted with ethyl acetate (80 mL). The resulting solution was washed successively with saturated aqueous sodium bicarbonate, water, and brine, dried over magnesium sulfate then concentrated in vacuo. The concentrate was purified by chromatography [Silica Gel, ethyl acetate: hexanes (1:3)→(1:2)] to afford compound 2 (117 mg, 54%). 1H NMR (CDCl3) δ 8.8 (d, 1H), 8.55 (m, 1H), 8.35 (d, 1H), 7.85 (dd, 1H), 7.65 (dd, 1H), 7.35 (m, 1H), 6.95 (d, 1H), 6.25 (br s, 2H).
example 2
4-Pyridin-3-yl-benzene-1,2-diamine (3): To a solution of compound 2 (117 mg, 0.54 mmol) in ethyl acetate (13 mL) was added 10% palladium on carbon (51 mg). The resulting suspension was placed in a Parr hydrogenation apparatus under 40 psi hydrogen gas while shaking at ambient temperature for 2 hours. The catalyst was removed by filtration and the filtrate concentrated in vacuo to afford compound 2 (115 mg, quantitative yield). 1H NMR (CDCl3) δ 8.8 (m, 1H), 8.45 (m, 1H), 7.75 (m, 1H), 7.25 (m, 1H), 6.95 (m, 2H), 6.80 (m, 1H), 3.25 (br s, 4H).
example 3
(5-Pyridin-3-yl-1H-benzoimidazol-2-yl)-carbamic acid ethyl ester (Ia-11): To a solution of 2-methyl-2-thiopseudourea (151 mg, 0.54 mmol) and ethylchloroformate (0.103 mL, 1.08 mmol) in water (2 mL) at 0° C. was added 25% aqueous sodium hydroxide in a dropwise fashion over 1 hour until the pH stabilized at 8. Enough acetic acid was then added to achieve pH 5 then sodium acetate trihydrate (74 mg, 0.54 mmol) and a solution of 2 (0.54 mmol) in ethanol (3 mL) were added. A catalytic amount of p-toluenesulfonic acid was added and the resulting mixture was heated at reflux for 1 hour. The reaction mixture was then cooled to ambient temperature and diluted with ethyl acetate (50 mL). The organic solution was washed with aqueous NaOH, water, and brine then dried over magnesium sulfate and concentrated in vacuo. The crude product was purified by preparative HPLC to afford compound Ia-11. 1H NMR (CDCl3) δ 9.1 (s, 1H), 8.75 (d, 1H), 8.5 (d, 1H), 7.9 (s, 1H), 7.8 (m, 1H), 7.65 (m, 2H), 4.3 (q, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| resistance | aaaaa | aaaaa |
| Antibiotic Resistance | aaaaa | aaaaa |
| antibiotic resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com