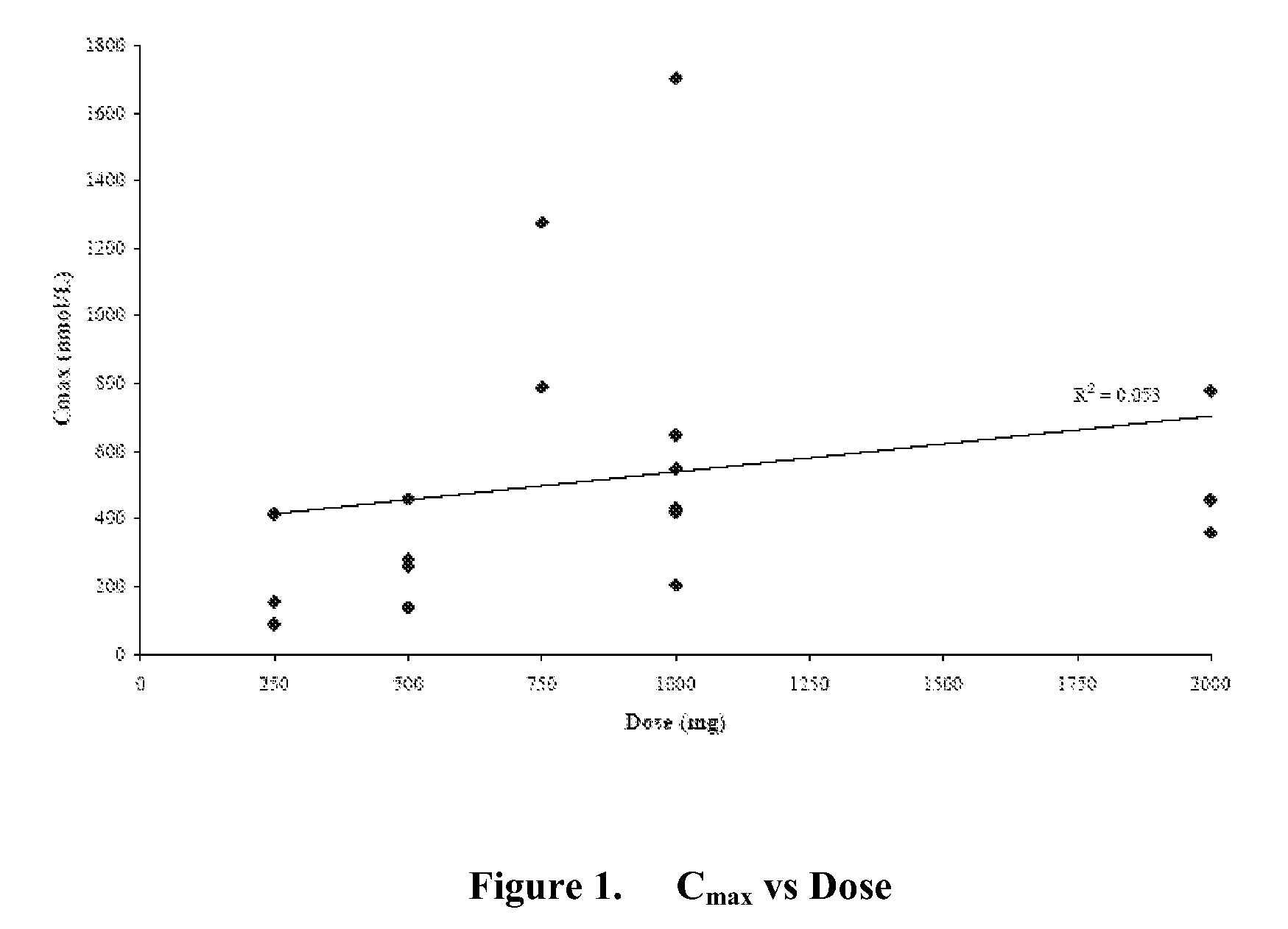
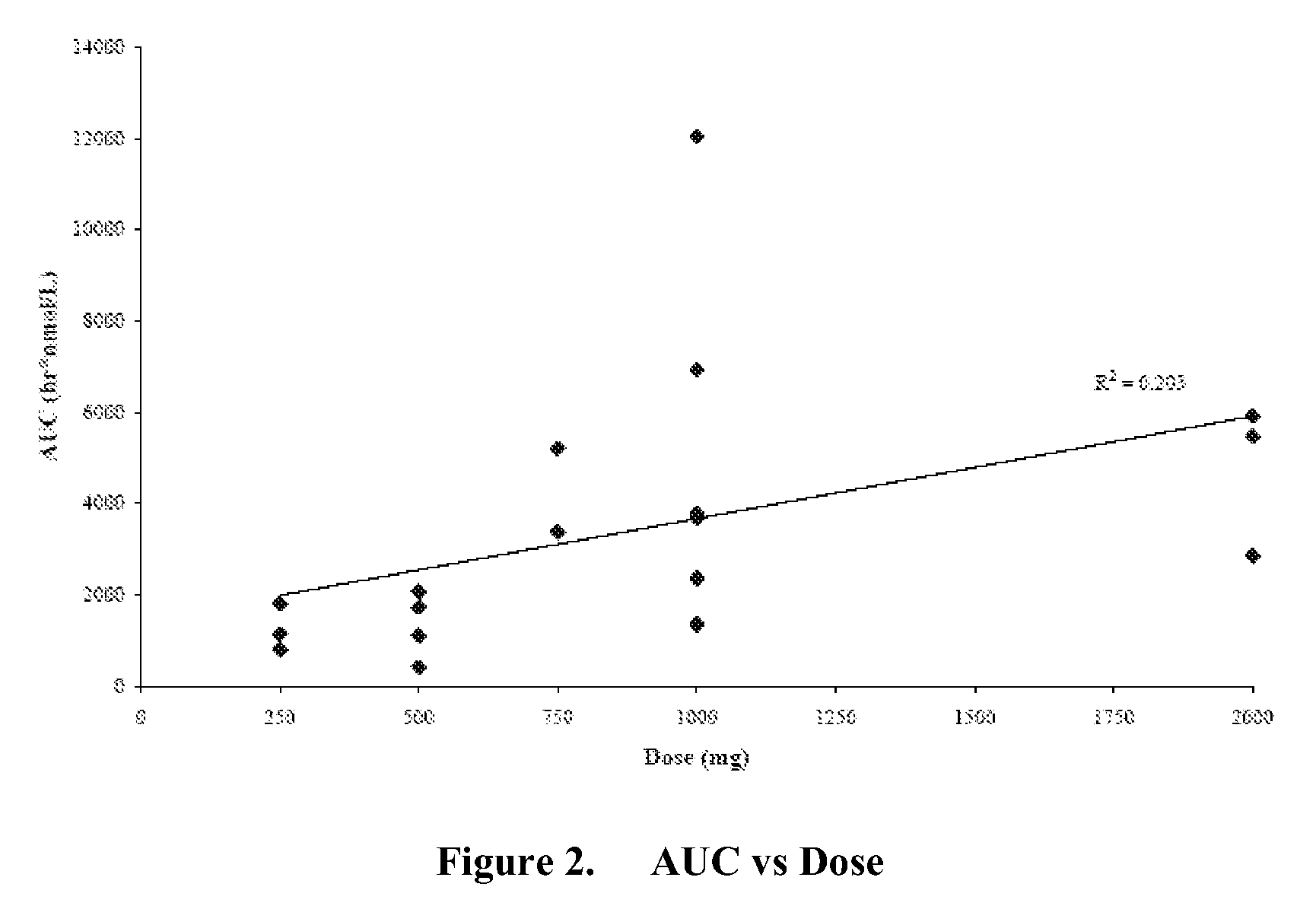
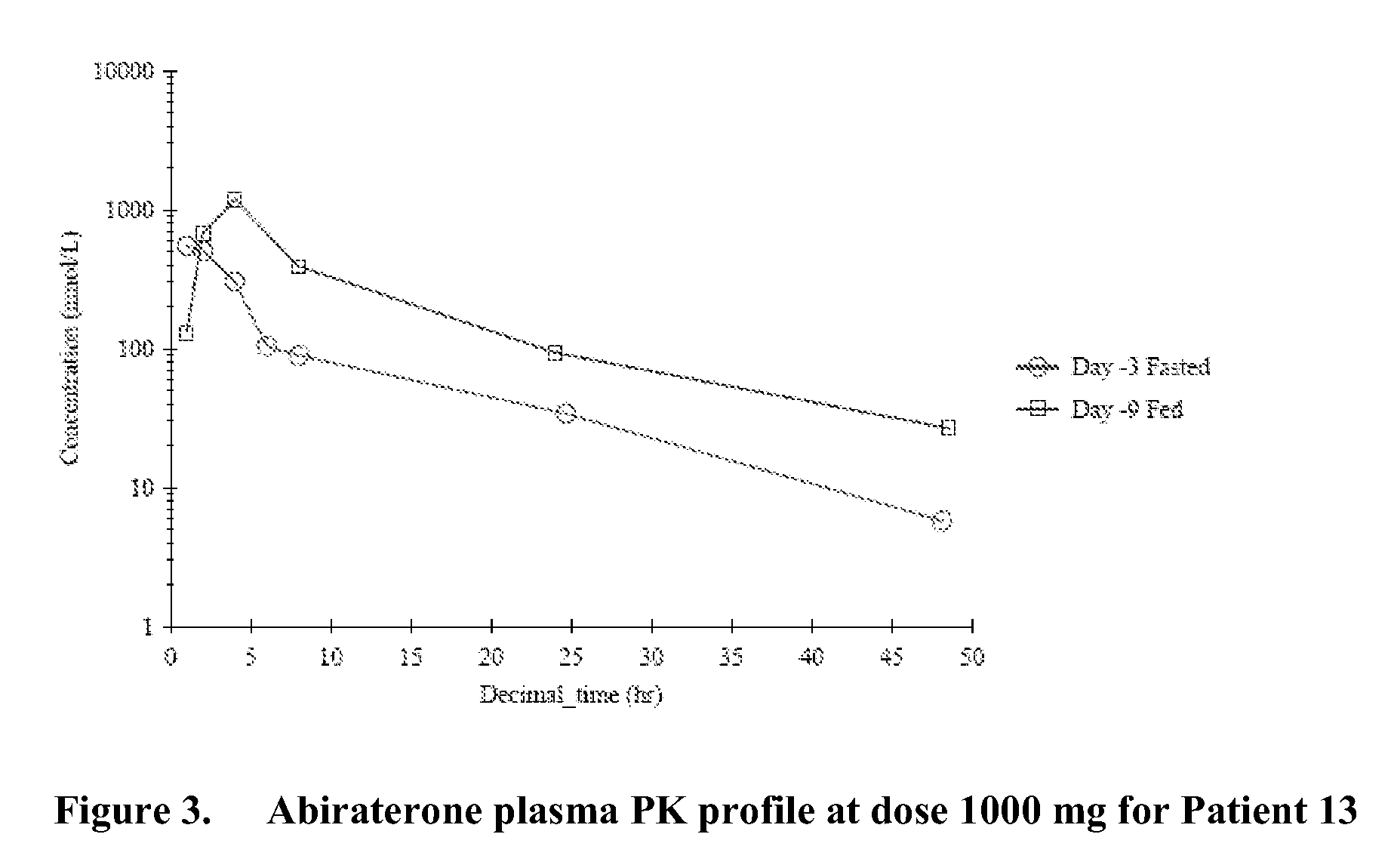
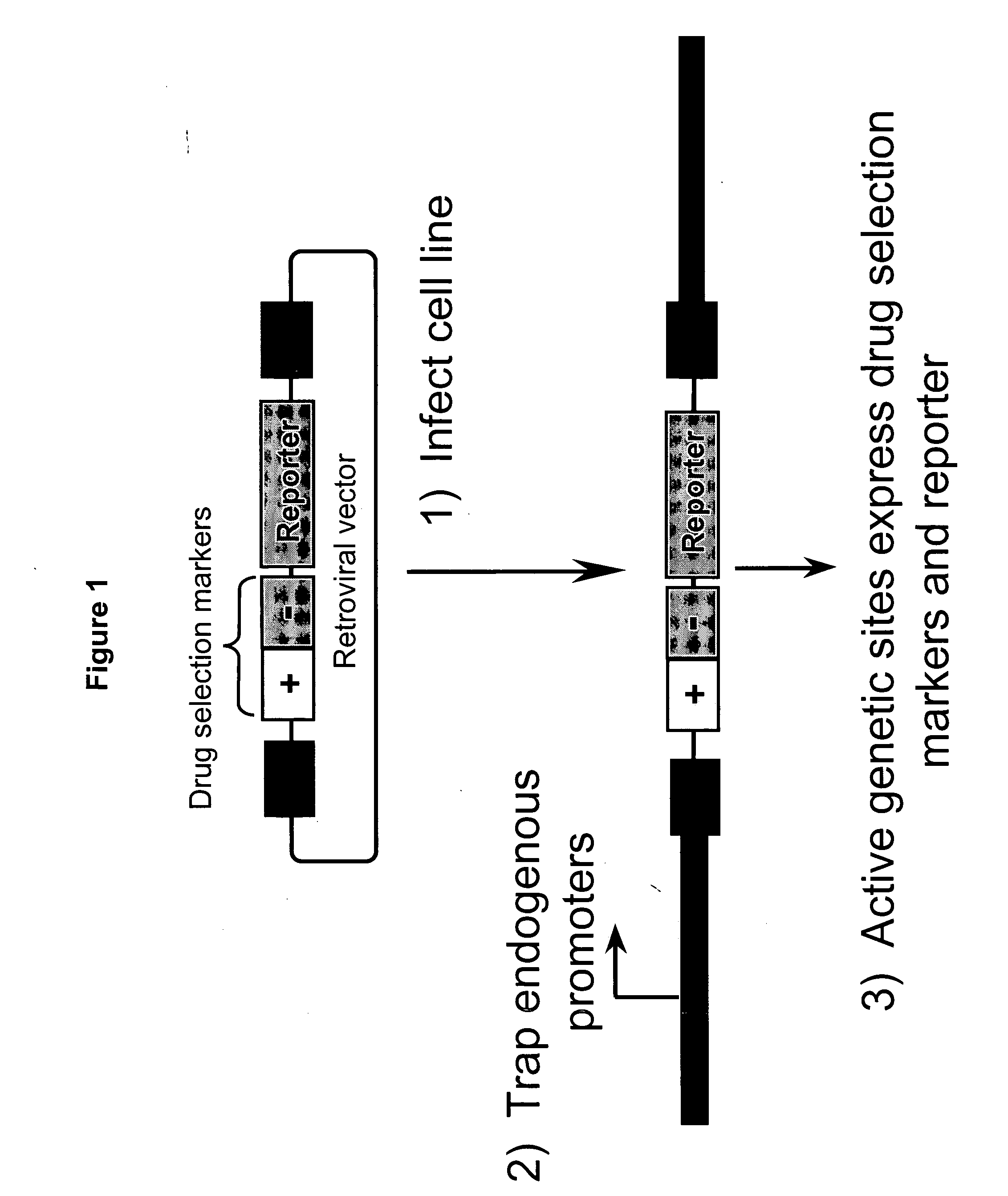
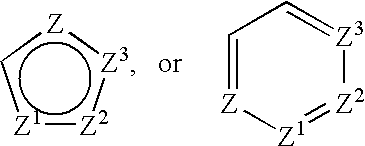Patents
Literature
95 results about "Enzyme Inhibitor Therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Biological markers predictive of anti-cancer response to epidermal growth factor receptor kinase inhibitors
InactiveUS20070212738A1Restore sensitivityHigh sensitivityDisease diagnosisAntineoplastic agentsEpidermal Growth Factor Receptor KinaseKinase
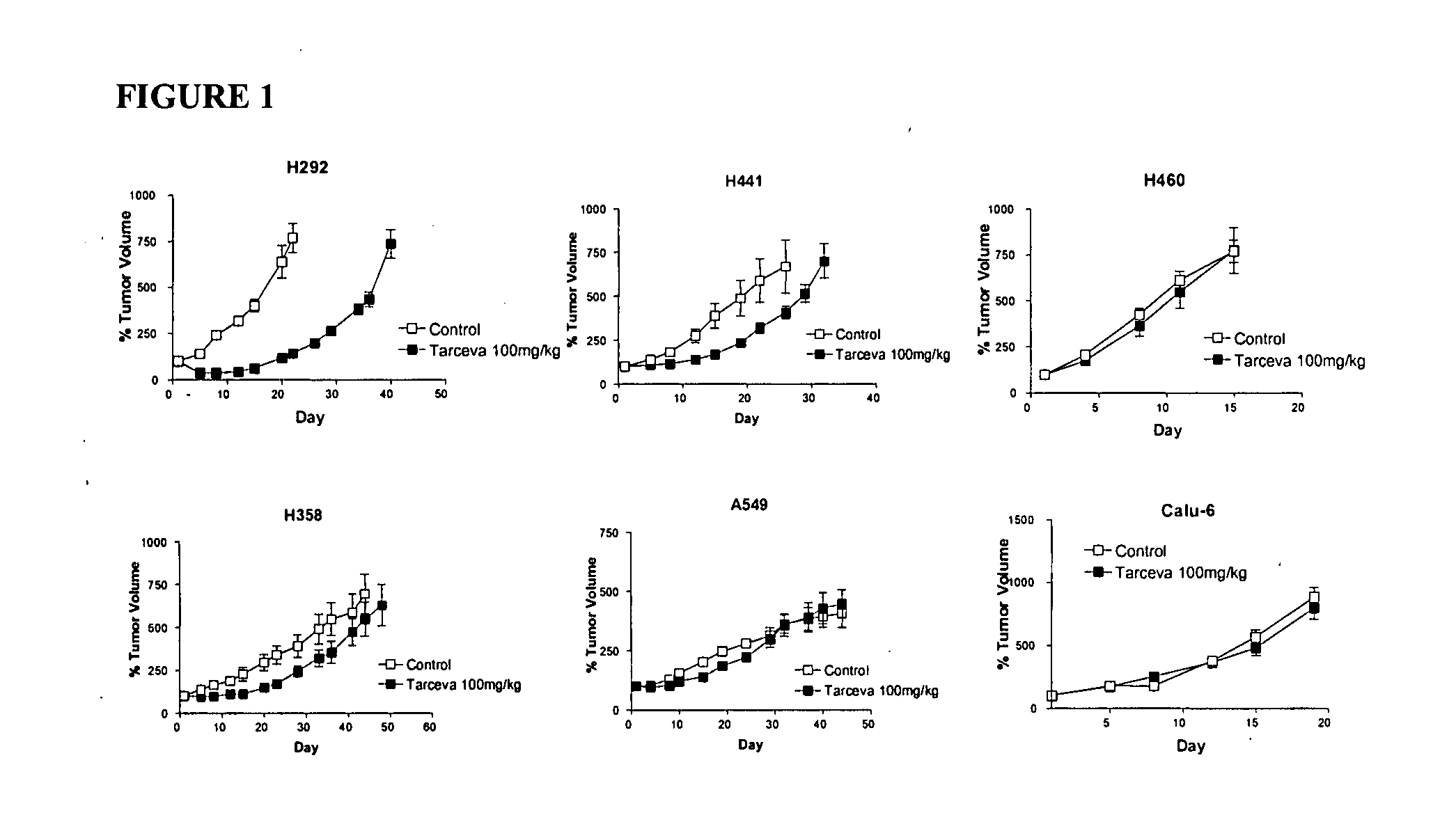
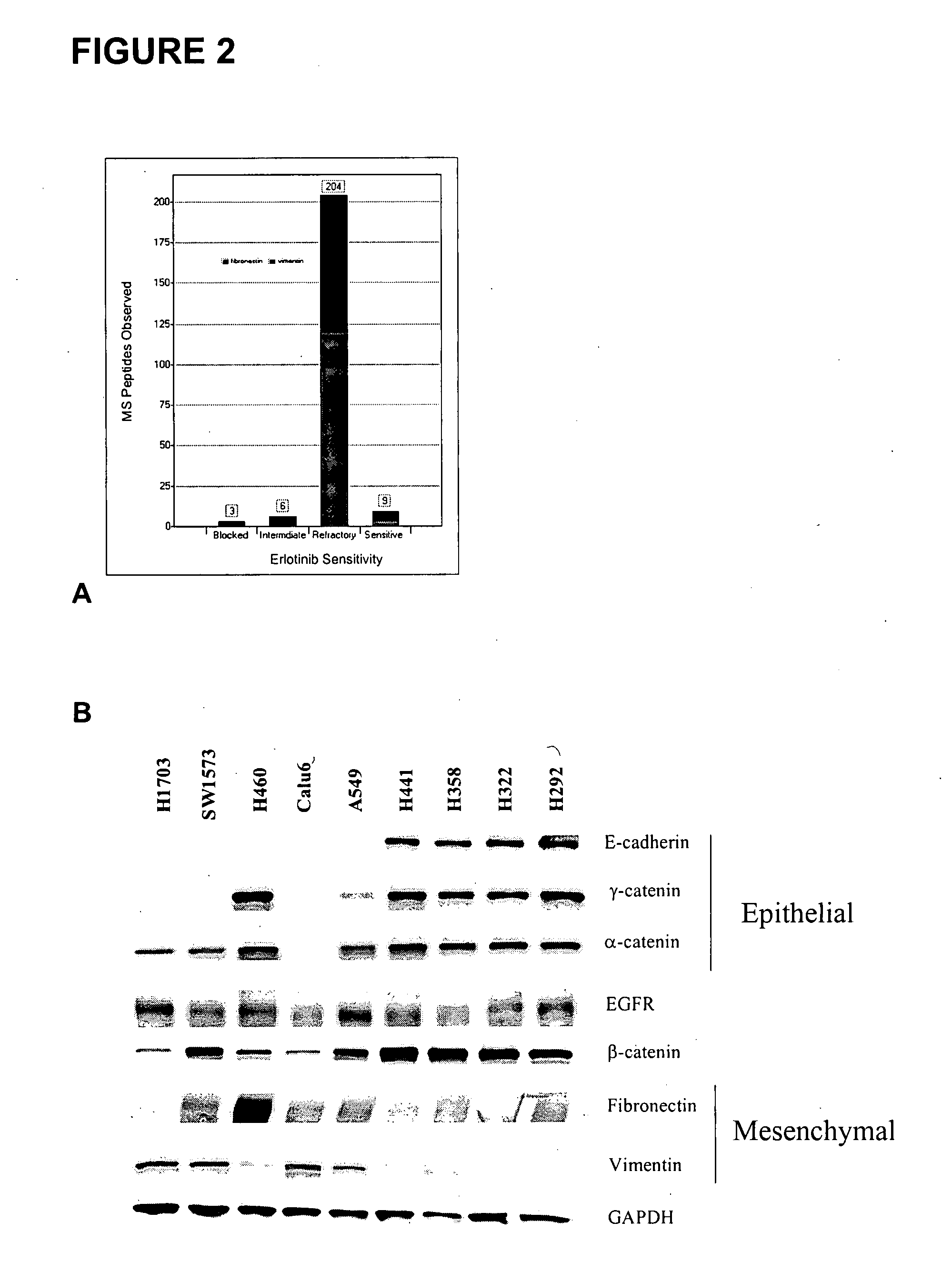
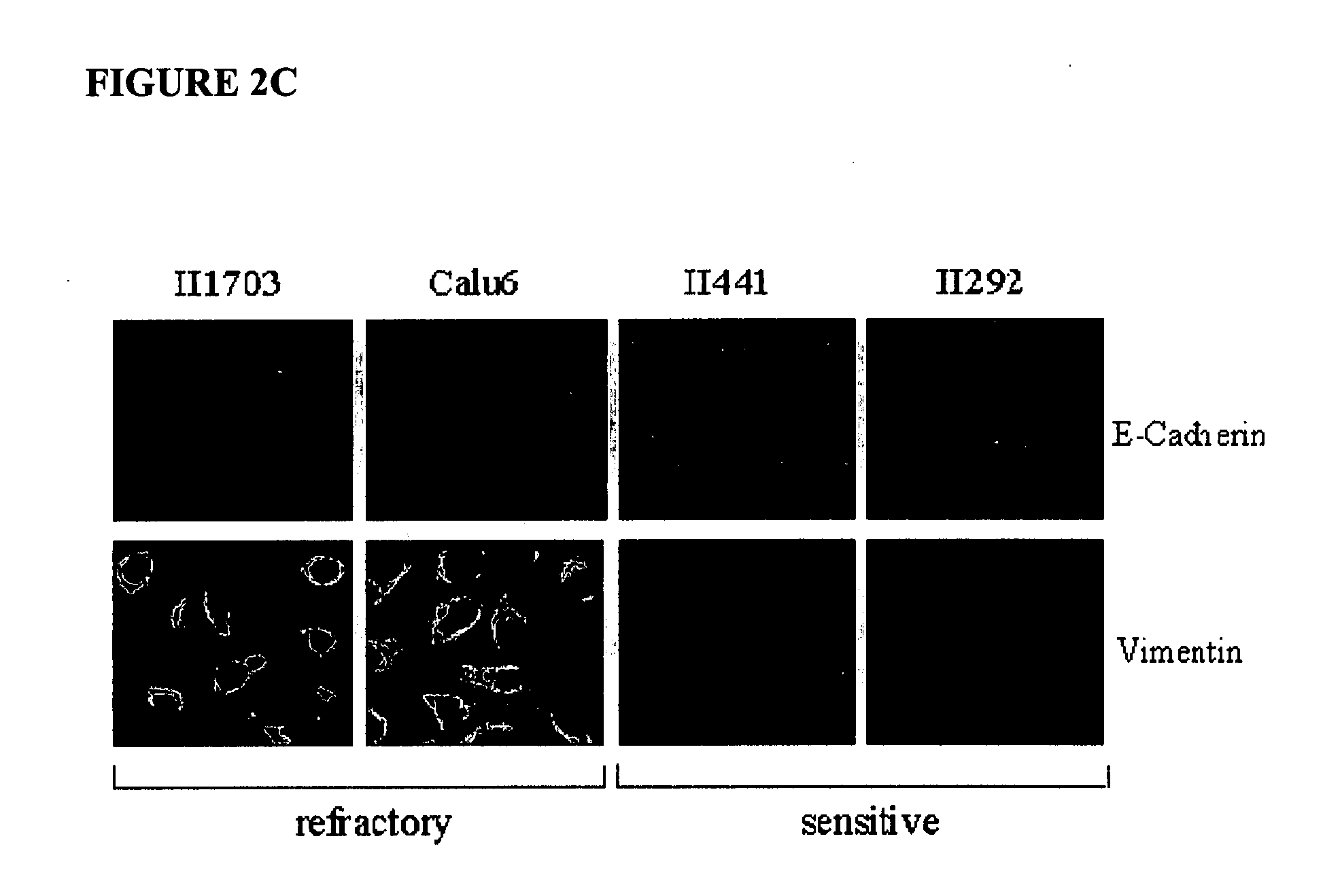
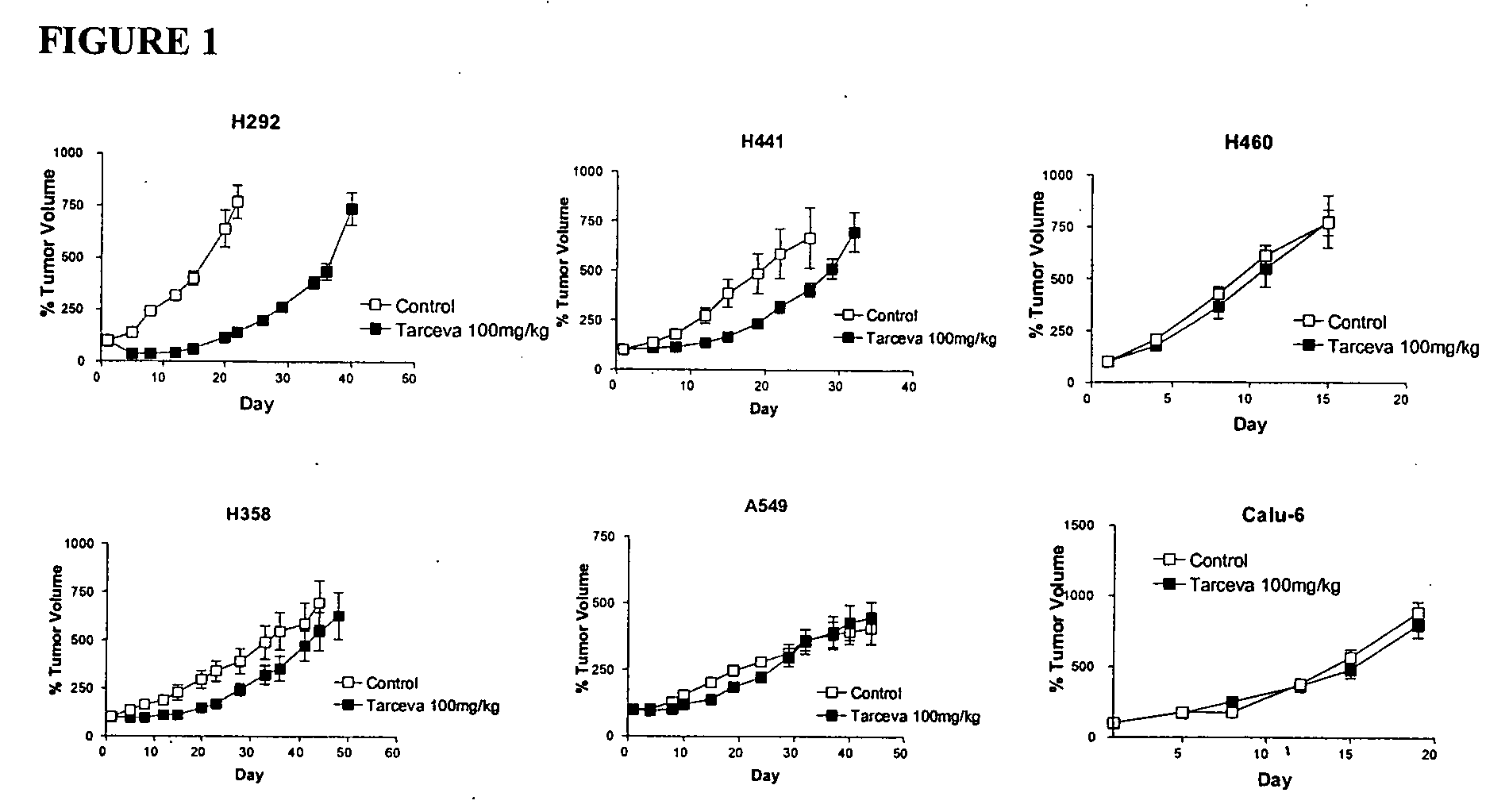
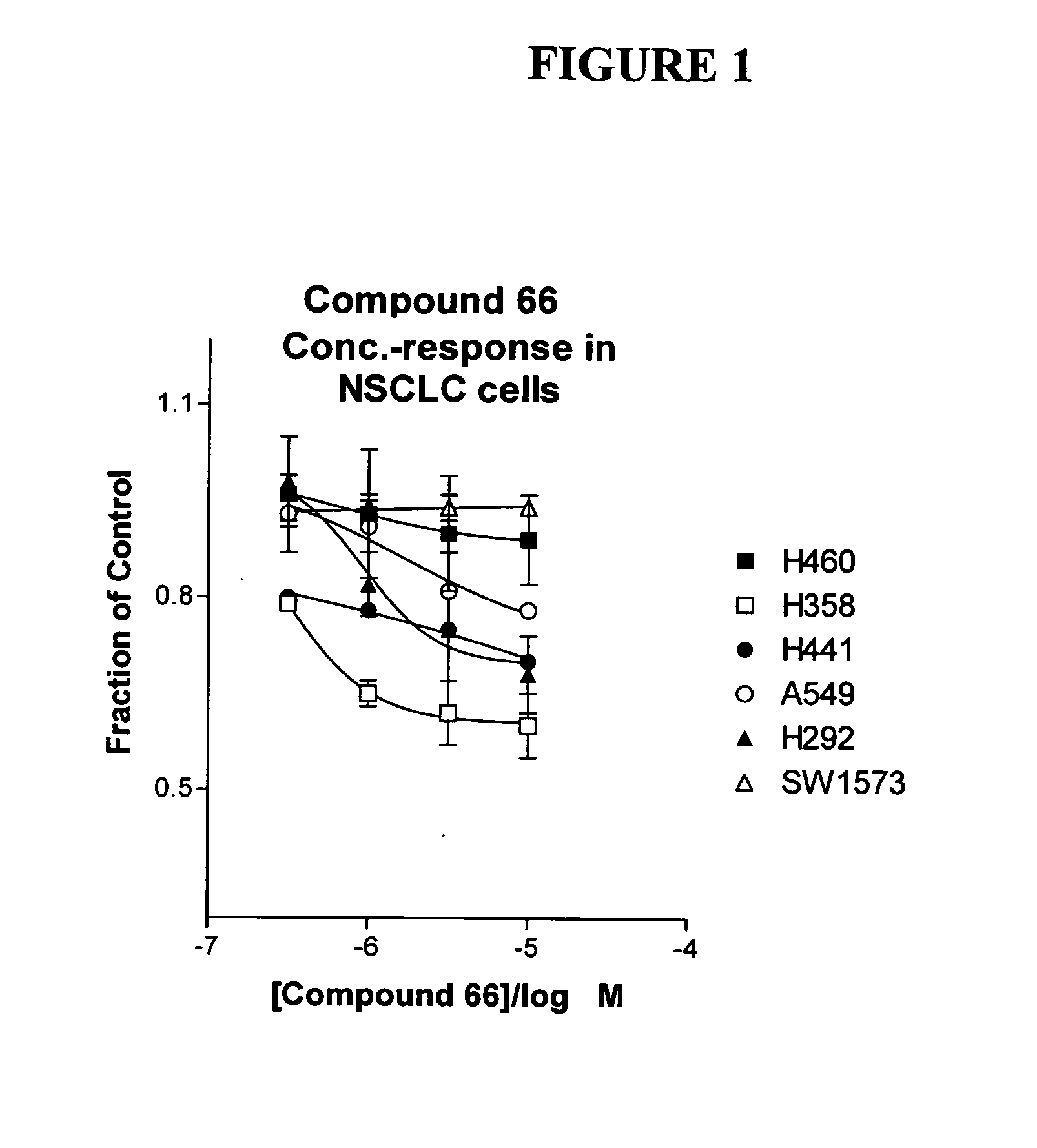
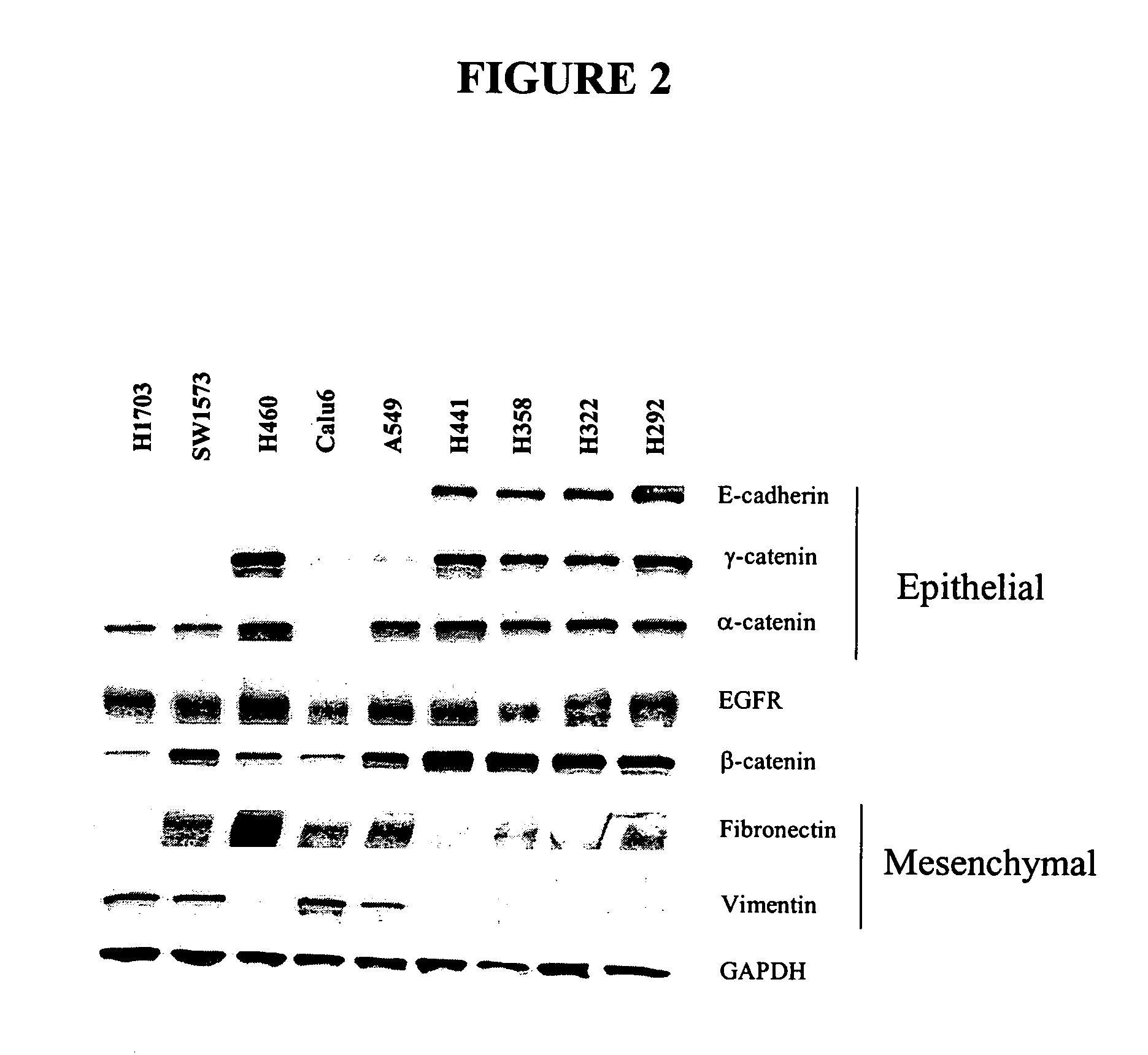
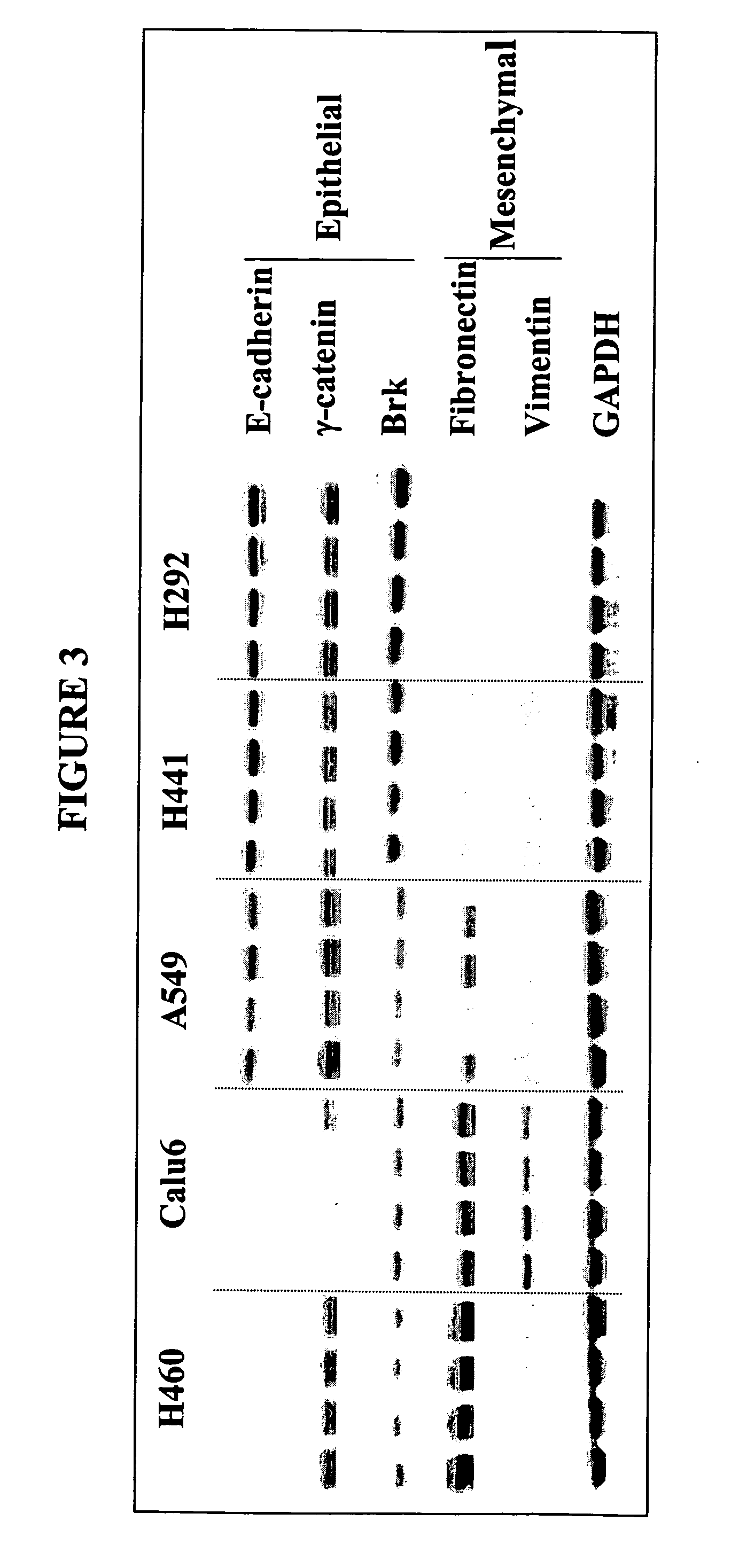
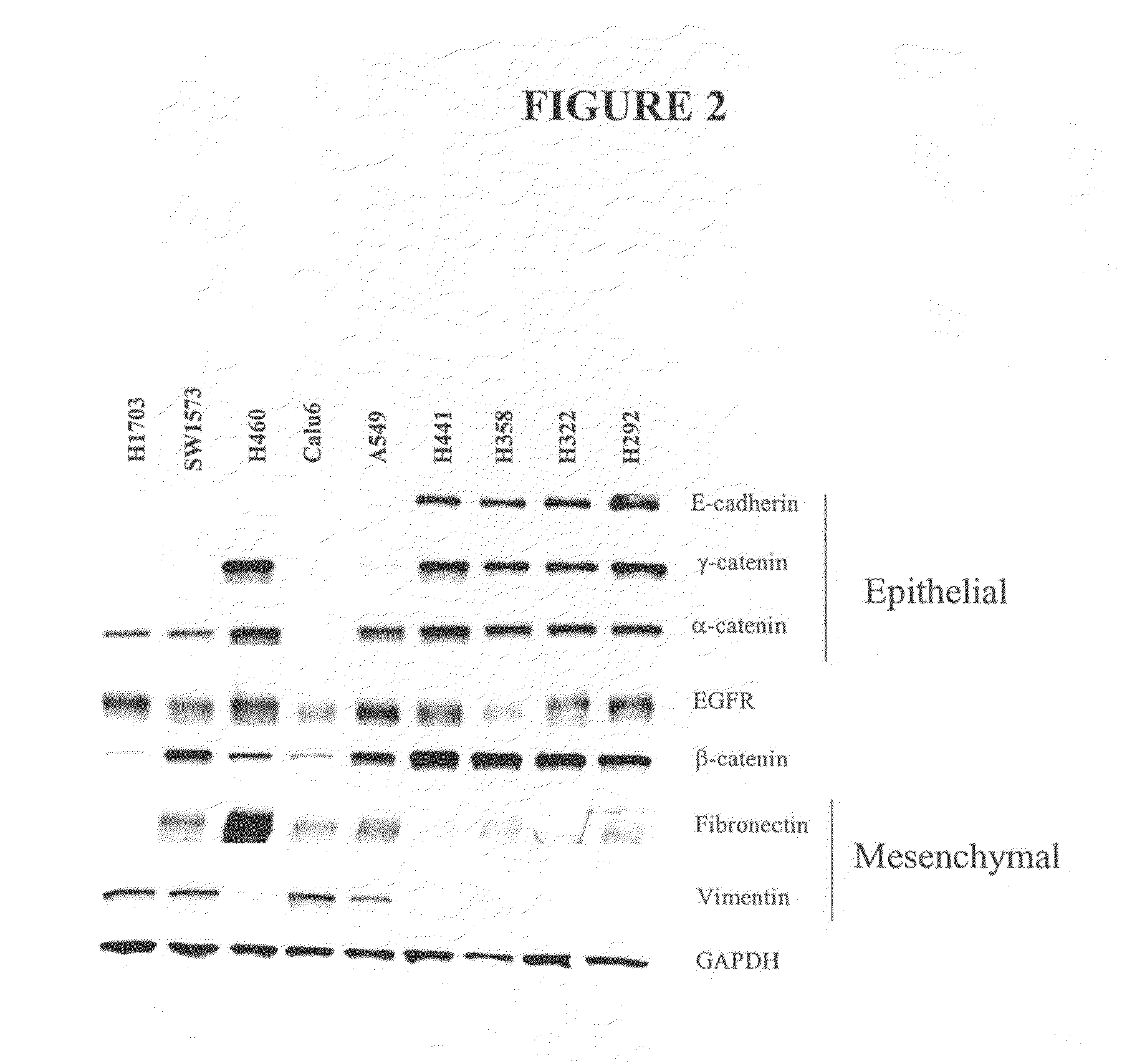
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with an EGFR kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an EGFR kinase inhibitor, comprising assessing whether the tumor cell has undergone an epithelial to mesenchymal transition (EMT), by determining the expression level of epithelial and / or mesenchymal biomarkers, wherein tumor cells that have undergone an EMT are substantially less sensitive to inhibition by EGFR kinase inhibitors. Improved methods for treating cancer patients with EGFR kinase inhibitors that incorporate the above methodology are also provided. Additionally, methods are provided for the identification of new biomarkers that are predictive of responsiveness of tumors to EGFR kinase inhibitors. Furthermore, methods for the identification of agents that restore the sensitivity of tumor cells that have undergone EMT to inhibition by EGFR kinase inhibitors are also provided.
Owner:OSI PHARMA LLC
Biological markers predictive of anti-cancer response to epidermal growth factor receptor kinase inhibitors
ActiveUS20060211060A1Restore sensitivityHigh sensitivityOrganic active ingredientsDisease diagnosisEpidermal Growth Factor Receptor KinaseKinase
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with an EGFR kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an EGFR kinase inhibitor, comprising assessing whether the tumor cell has undergone an epithelial to mesenchymal transition (EMT), by determining the expression level of epithelial and / or mesenchymal biomarkers, wherein tumor cells that have undergone an EMT are substantially less sensitive to inhibition by EGFR kinase inhibitors. Improved methods for treating cancer patients with EGFR kinase inhibitors that incorporate the above methodology are also provided. Additionally, methods are provided for the identification of new biomarkers that are predictive of responsiveness of tumors to EGFR kinase inhibitors. Furthermore, methods for the identification of agents that restore the sensitivity of tumor cells that have undergone EMT to inhibition by EGFR kinase inhibitors are also provided.
Owner:OSI PHARMA INC
Method for treating diseases using HSP90-inhibiting agents in combination with enzyme inhibitors
The present invention provides a method for treating cancer. The method involves the administration of an HSP90 inhibitor and an enzyme inhibitor, where the combined administration provides a synergistic effect. In one aspect of the invention, a method of treating cancer is provided where a subject is treated with a dose of an HSP90 inhibitor in one step and a dose of an enzyme inhibitor in another step. In another aspect of the invention, a method of treating cancer is provided where a subject is first treated with a dose of an HSP90 inhibitor and subsequently treated with a dose of an enzyme inhibitor. In another aspect of the invention, a method of treating cancer is provided where a subject is first treated with a dose of an enzyme inhibitor and subsequently treated with a dose of an HSP90 inhibitor.
Owner:KOSAN BIOSCI
Glucosylceramide synthase inhibitors and therapeutic methods using the same
Glucosylceramide synthase inhibitors and compositions containing the same are disclosed. Methods of using the glucosylceramide synthase inhibitors in the treatment of diseases and conditions wherein inhibition of glucosylceramide synthase provides a benefit, like Gaucher disease and Fabry disease, also are disclosed.
Owner:RGT UNIV OF MICHIGAN
Biological markers predictive of Anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors
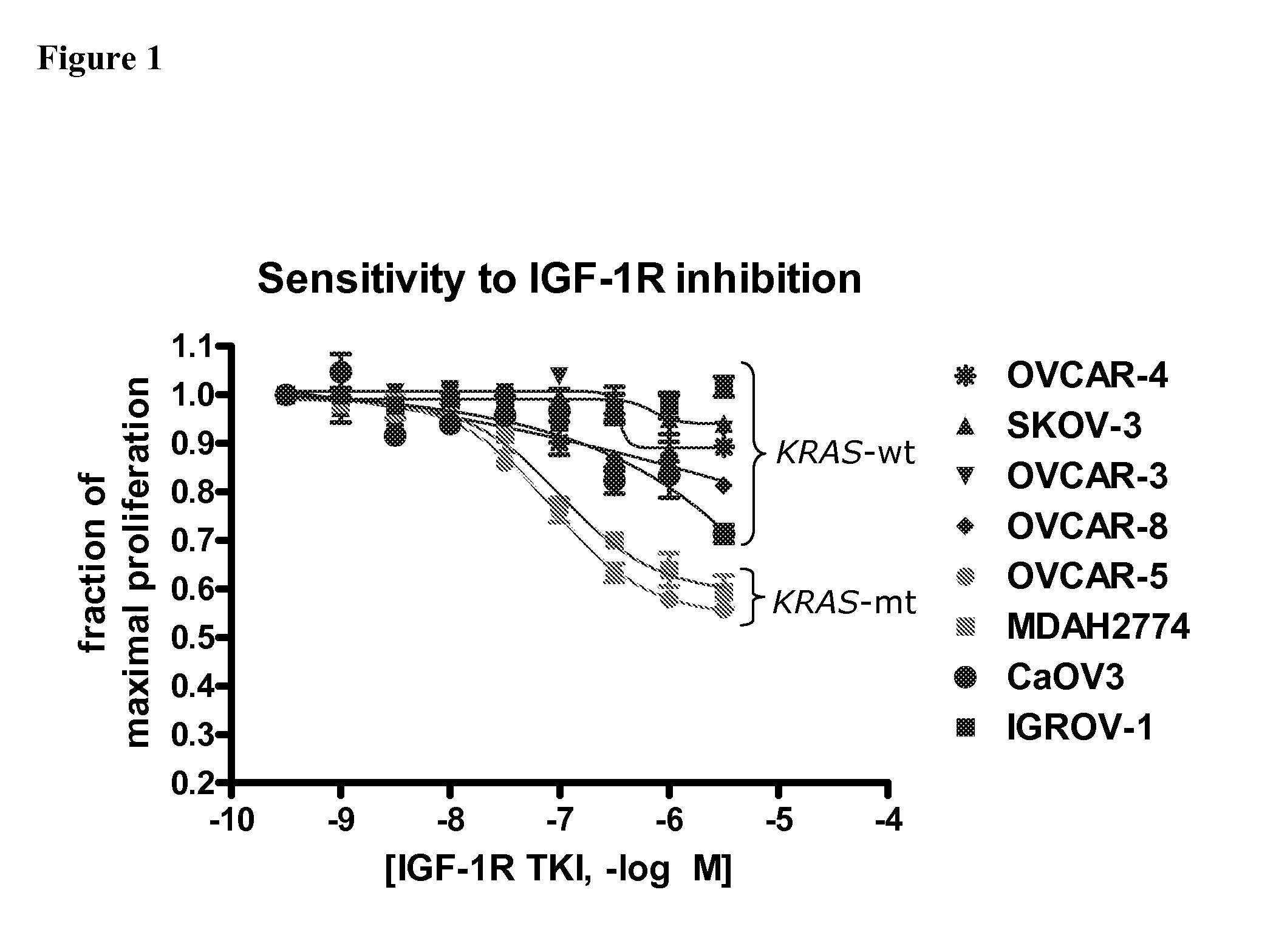
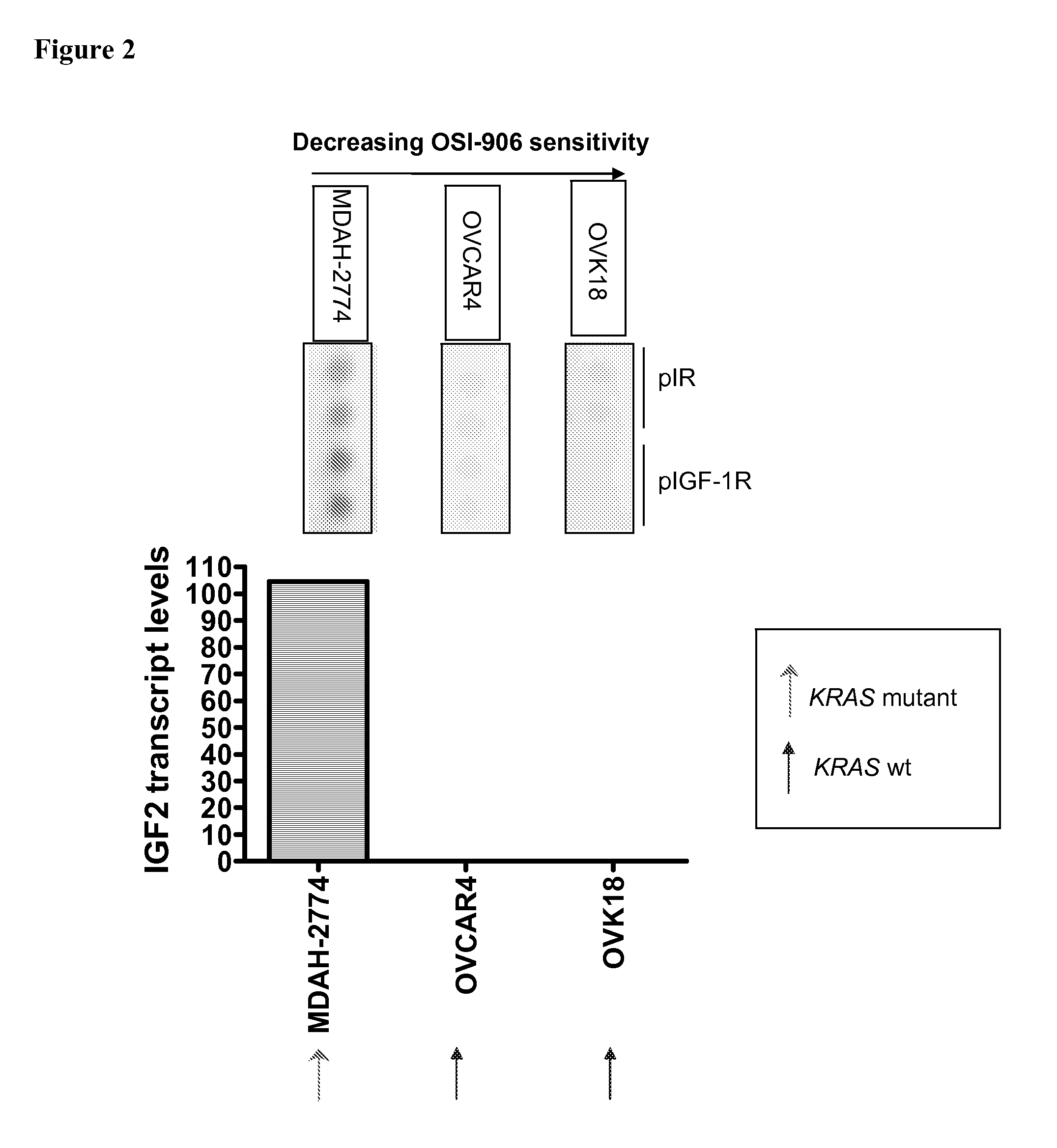
The present invention provides diagnostic methods for predicting the effectiveness of treatment of an ovarian cancer patient with an IGF-1R kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an IGF-1R kinase inhibitor, comprising assessing whether the tumor cells possess mutant K-RAS. The present invention thus provides a method of identifying patients with ovarian cancer who are most likely to benefit from treatment with an IGF-1R kinase inhibitor. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate this methodology are also provided. The present invention also provides diagnostic methods for predicting the effectiveness of treatment of cancer patients with IGF-1R kinase inhibitors, based on a determination of the mutation status of the genes K-RAS, B-RAF, PTEN and PIK3CA, which can be used to identify tumor cell types that will be sensitive to IGF-1R kinase inhibitors, and also those that will be insensitive.
Owner:OSI PHARMA INC
Predicitive biomarkers in cancer therapy
InactiveUS20070190583A1Increased pretreatment levelMicrobiological testing/measurementIn-vivo testing preparationsApoptosisPredictive biomarker
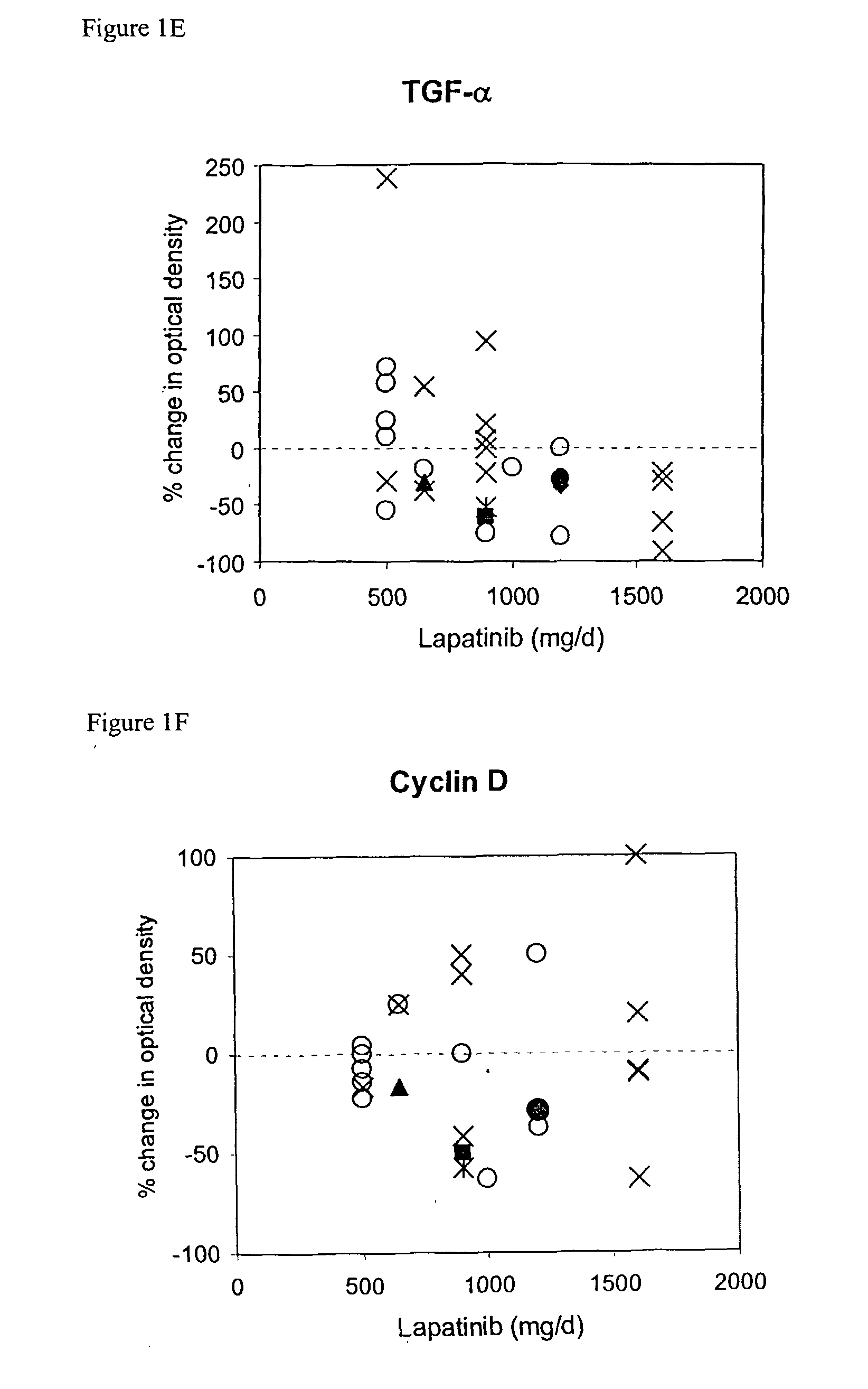
The use of various biomarkers to assess a subject's suitability for treatment with a EGFR / ErbB2 kinase inhibitor for a solid tumor are described. The biomarkers include TGFalpha, pS6, IGF-1R and levels of apoptosis occurring in tumor tissue.
Owner:NOVARTIS AG
Biological markers predictive of anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors
InactiveUS20090092596A1High level of biomarkerReduce sensitivityMicrobiological testing/measurementAntibody ingredientsCell growthWilms' tumor
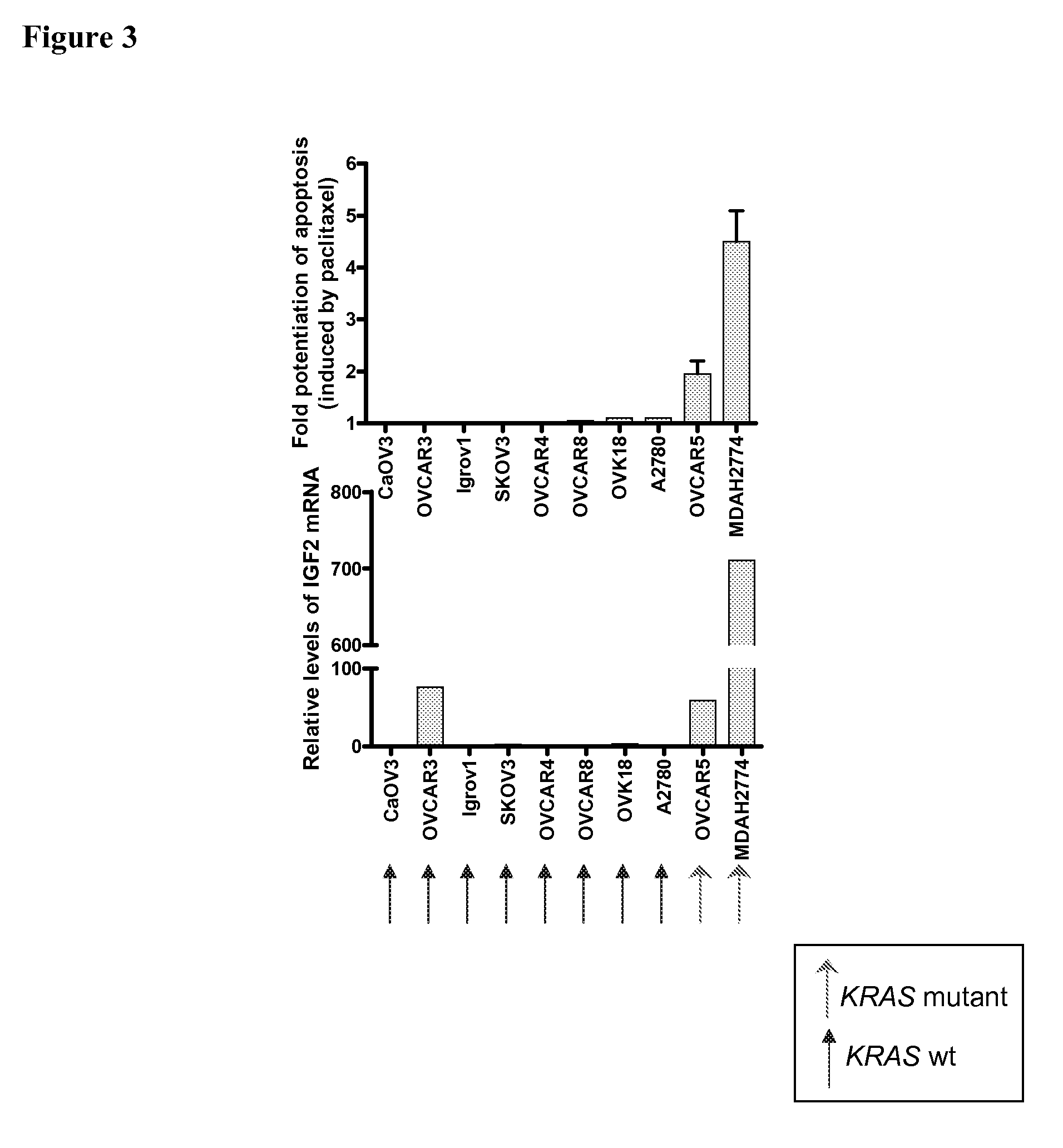
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with an IGF-1R kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an IGF-1R kinase inhibitor, comprising assessing whether the tumor cell has undergone an epithelial to mesenchymal transition (EMT), by determining the expression level of epithelial and / or mesenchymal biomarkers, wherein tumor cells that have undergone an EMT are substantially less sensitive to inhibition by IGF-1R kinase inhibitors. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate the above methodology are also provided. Additionally, methods are provided for the identification of new biomarkers that are predictive of responsiveness of tumors to IGF-1R kinase inhibitors. Furthermore, methods for the identification of agents that restore the sensitivity of tumor cells that have undergone EMT to inhibition by IGF-1R kinase inhibitors are also provided. pErk, HER3 and pHER are also demonstrated to be effective biomarkers for predicting sensitivity of tumor cells to IGF-1R kinase inhibitors.
Owner:OSI PHARMA INC
Substituted imidazo[1,2b]pyridazine compounds as trk kinase inhibitors
Compounds of formula (I): in which R1, R2, R3, R4, X, Y and n have the meanings given in the specification, are inhibitors of Trk kinases and are useful in the treatment of diseases which can be treated with a Trk kinase inhibitor. The formula (I) is shown in the description.
Owner:ARRAY BIOPHARMA
Biological markers predictive of anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors
ActiveUS20090093488A1High level of biomarkerReduce sensitivityBiocideNervous disorderAbnormal tissue growthCell growth
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with an IGF-1R kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an IGF-1R kinase inhibitor, comprising assessing whether the tumor cell has undergone an epithelial to mesenchymal transition (EMT), by determining the expression level of epithelial and / or mesenchymal biomarkers, wherein tumor cells that have undergone an EMT are substantially less sensitive to inhibition by IGF-1R kinase inhibitors. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate the above methodology are also provided. Additionally, methods are provided for the identification of new biomarkers that are predictive of responsiveness of tumors to IGF-1R kinase inhibitors. Furthermore, methods for the identification of agents that restore the sensitivity of tumor cells that have undergone EMT to inhibition by IGF-1R kinase inhibitors are also provided. pErk, HER3 and pHER are also demonstrated to be effective biomarkers for predicting sensitivity of tumor cells to IGF-1R kinase inhibitors.
Owner:OSI PHARMA INC
Biological markers predictive of anti-cancer response to kinase inhibitors
InactiveUS20080312260A1Reduce sensitivityHigh sensitivityBiocideOrganic active ingredientsReceptor Protein-Tyrosine KinasesRaf Kinase Inhibitor
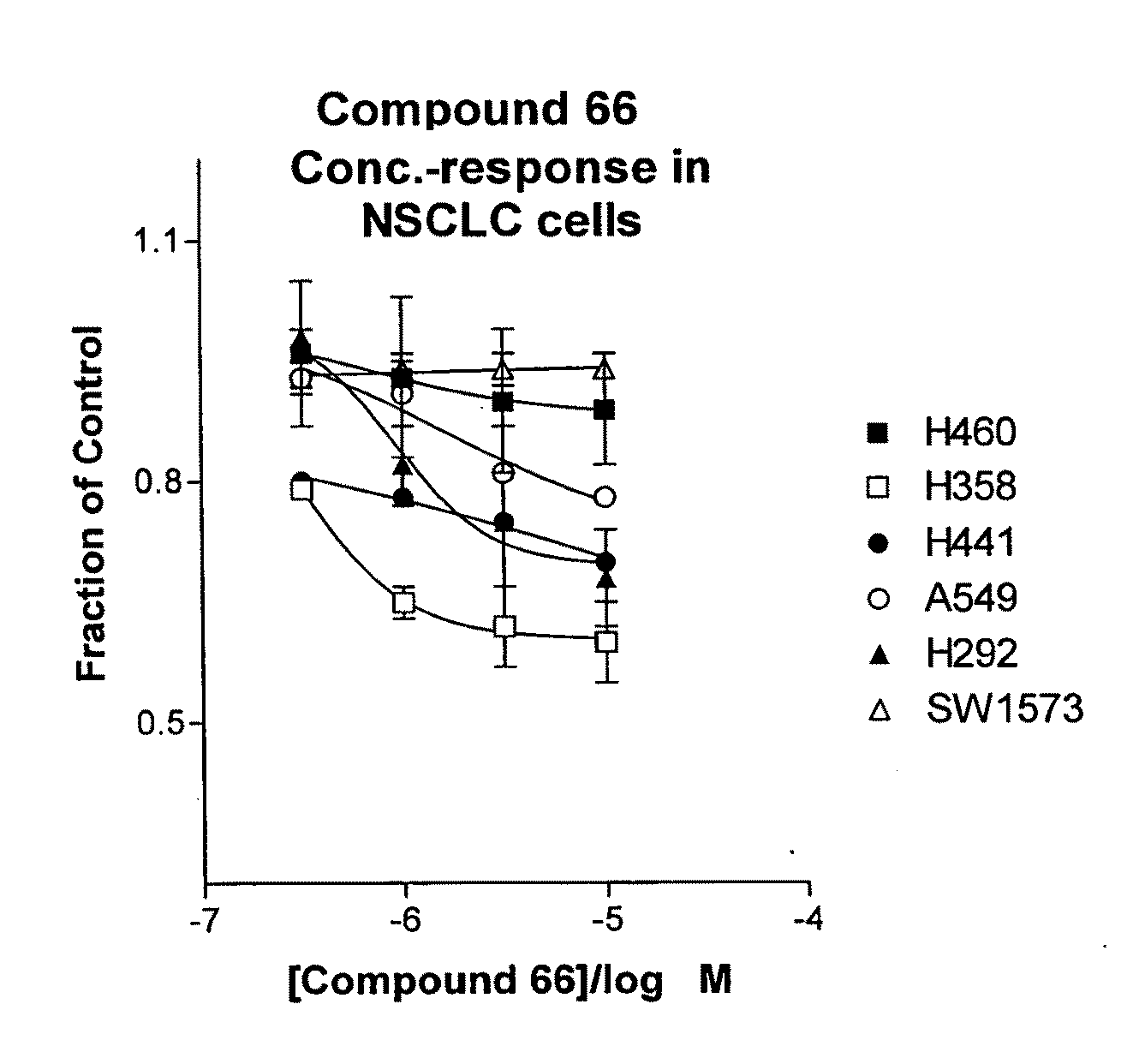
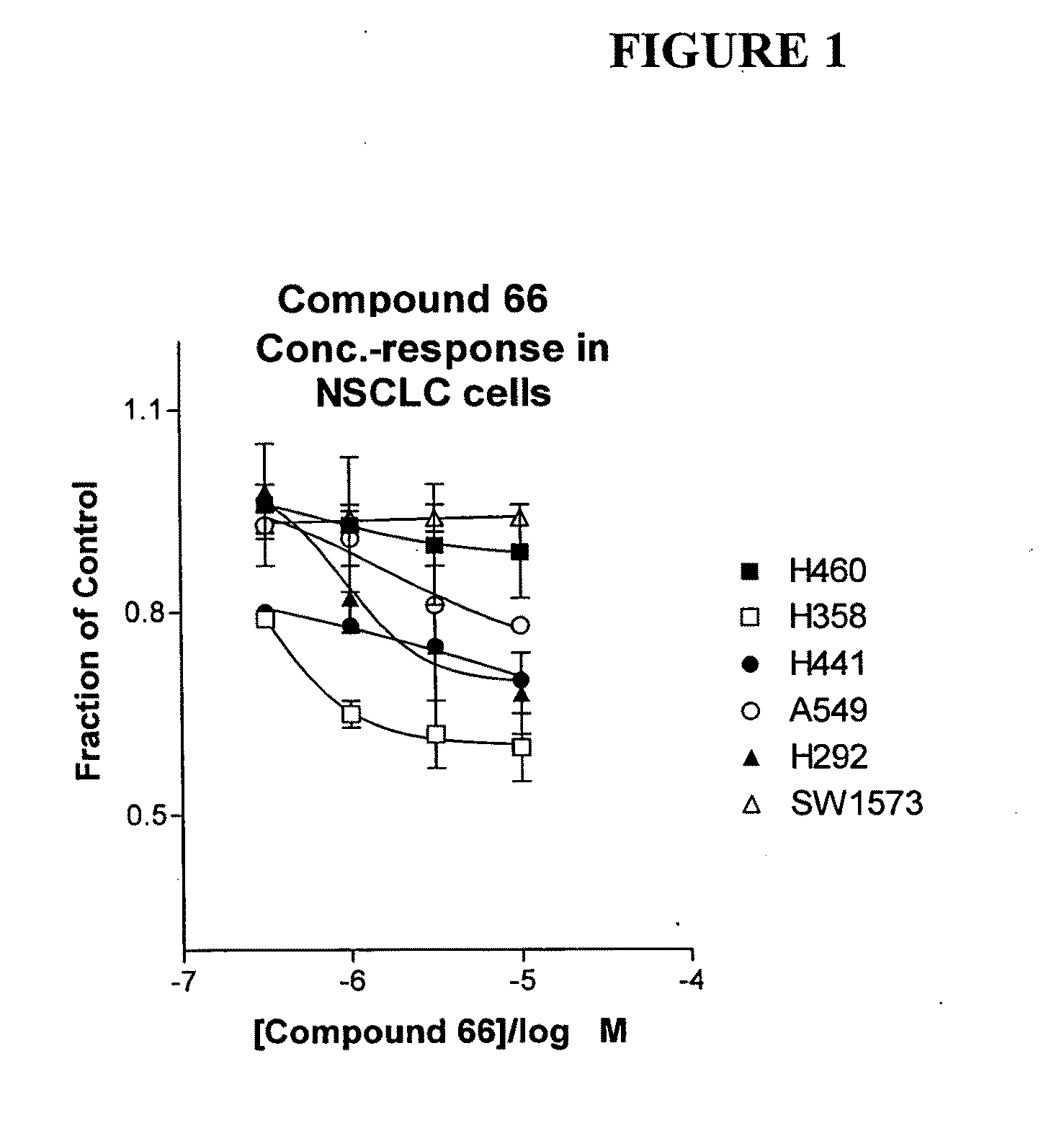
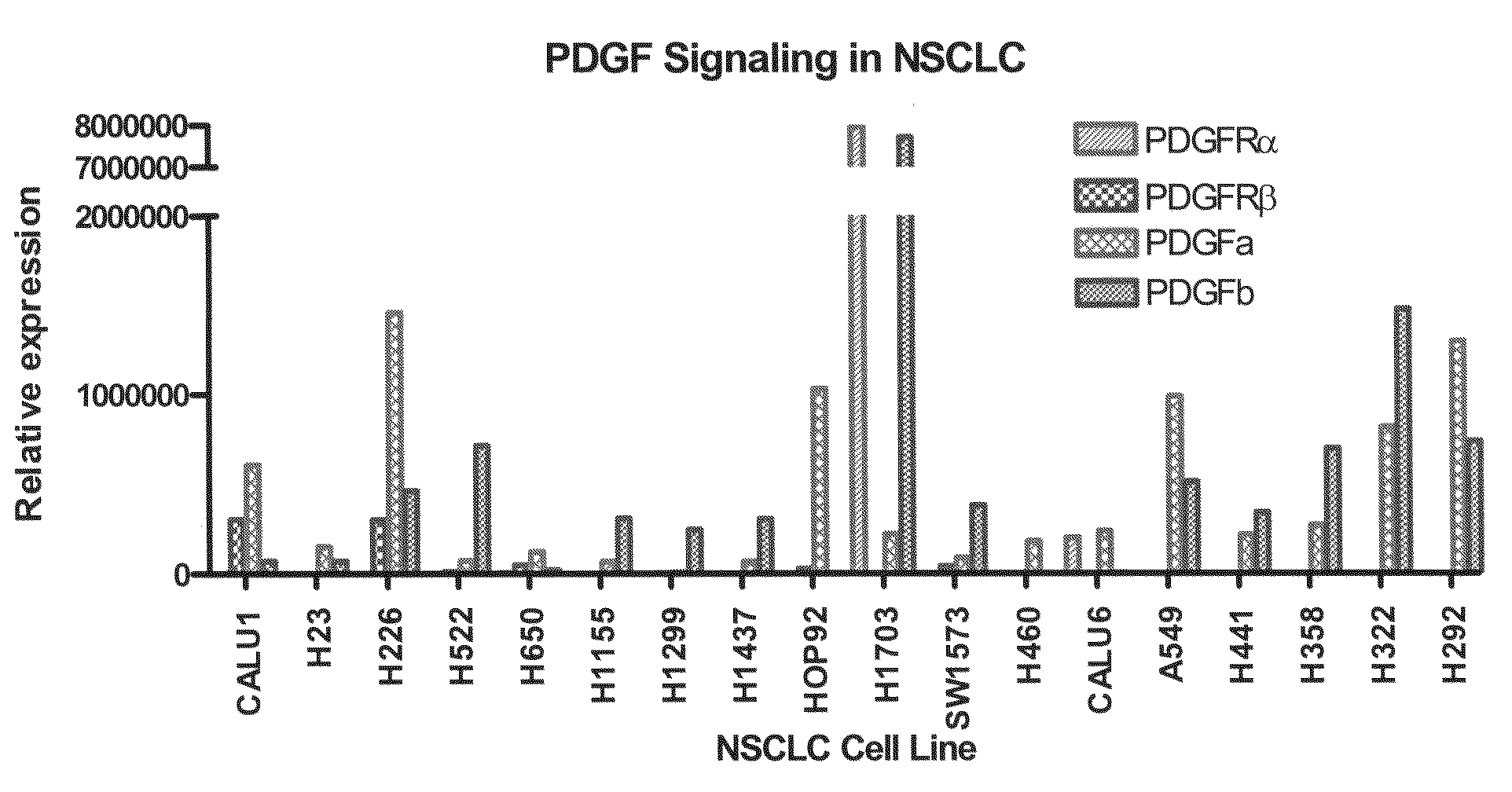
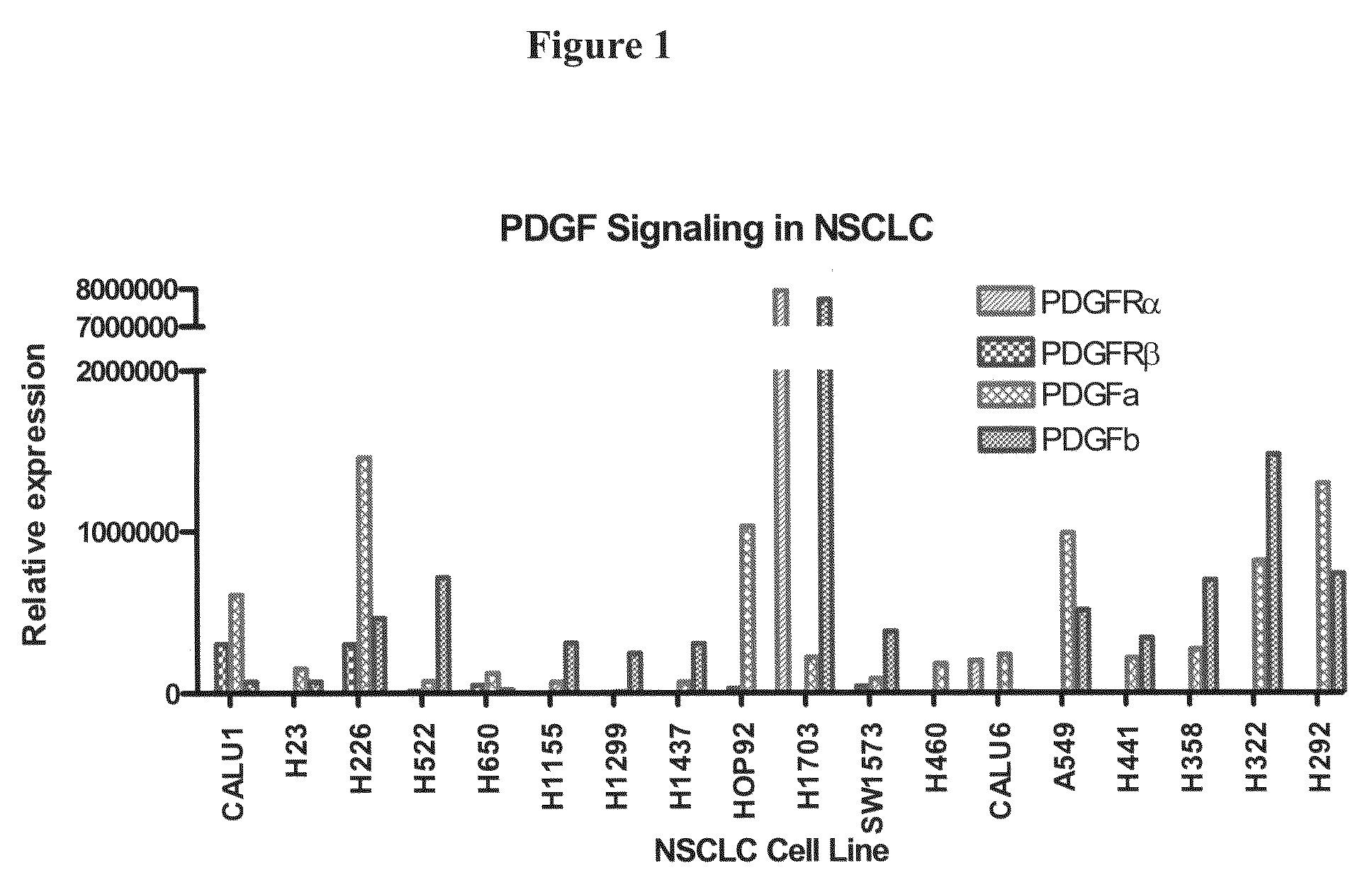
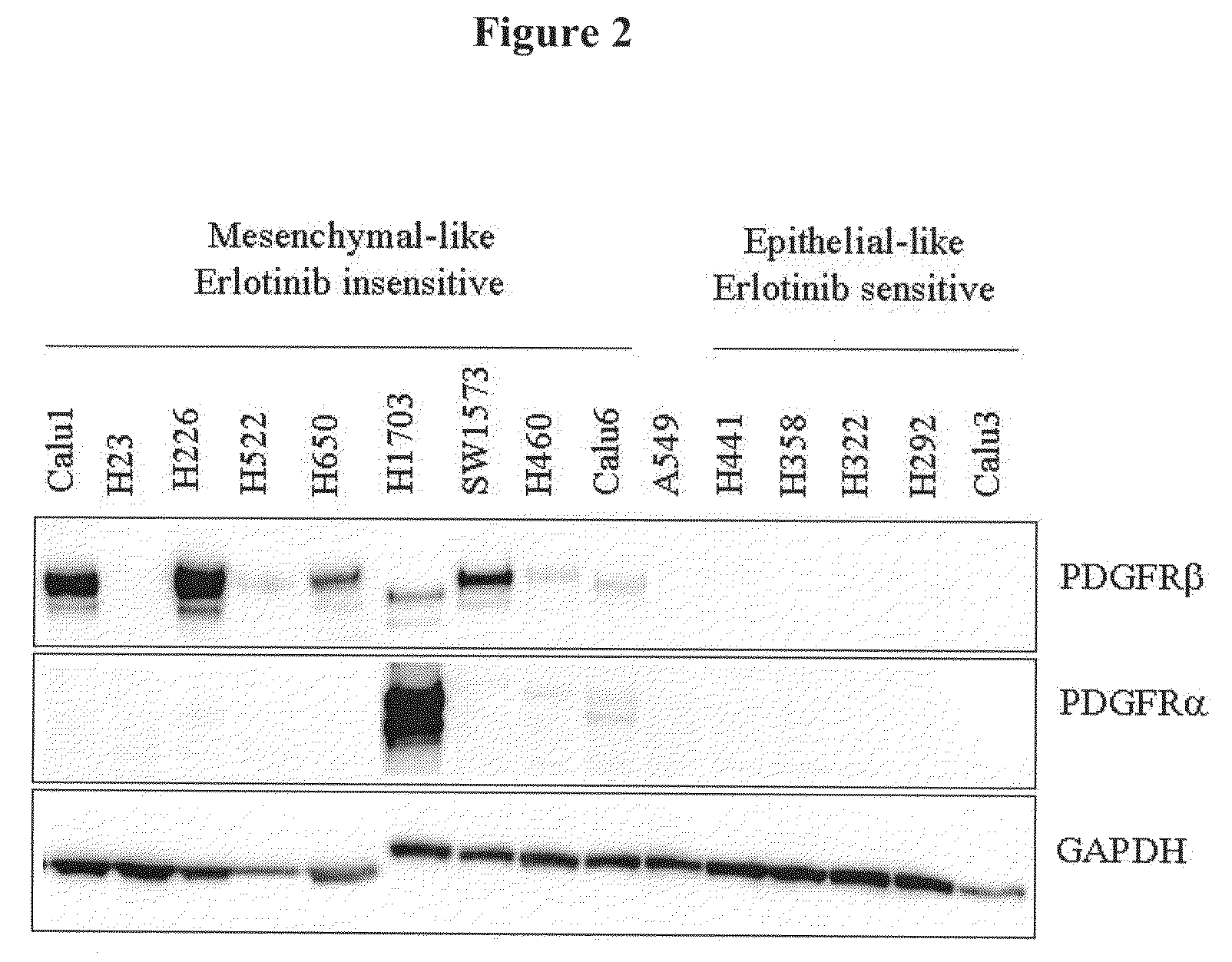
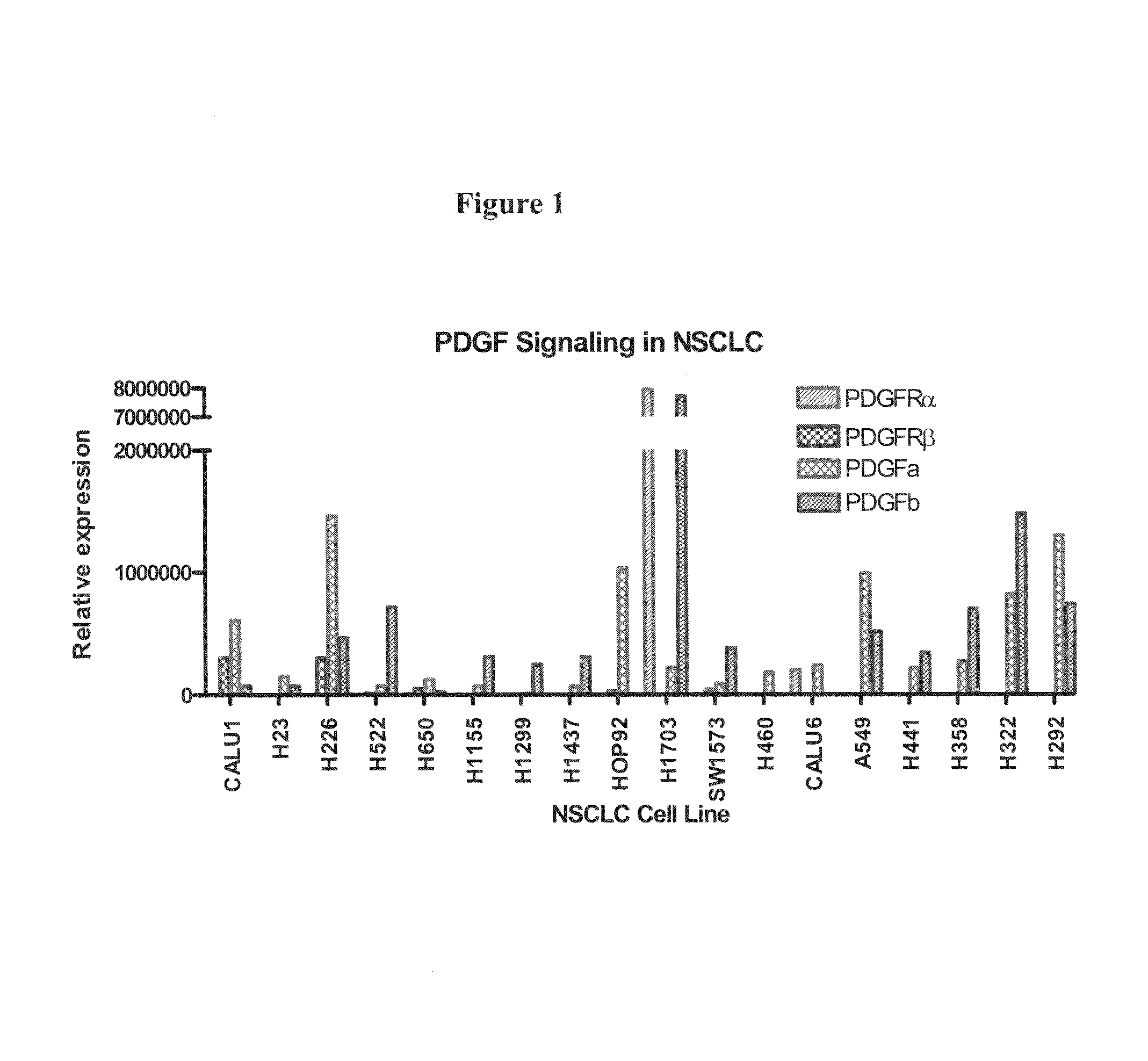
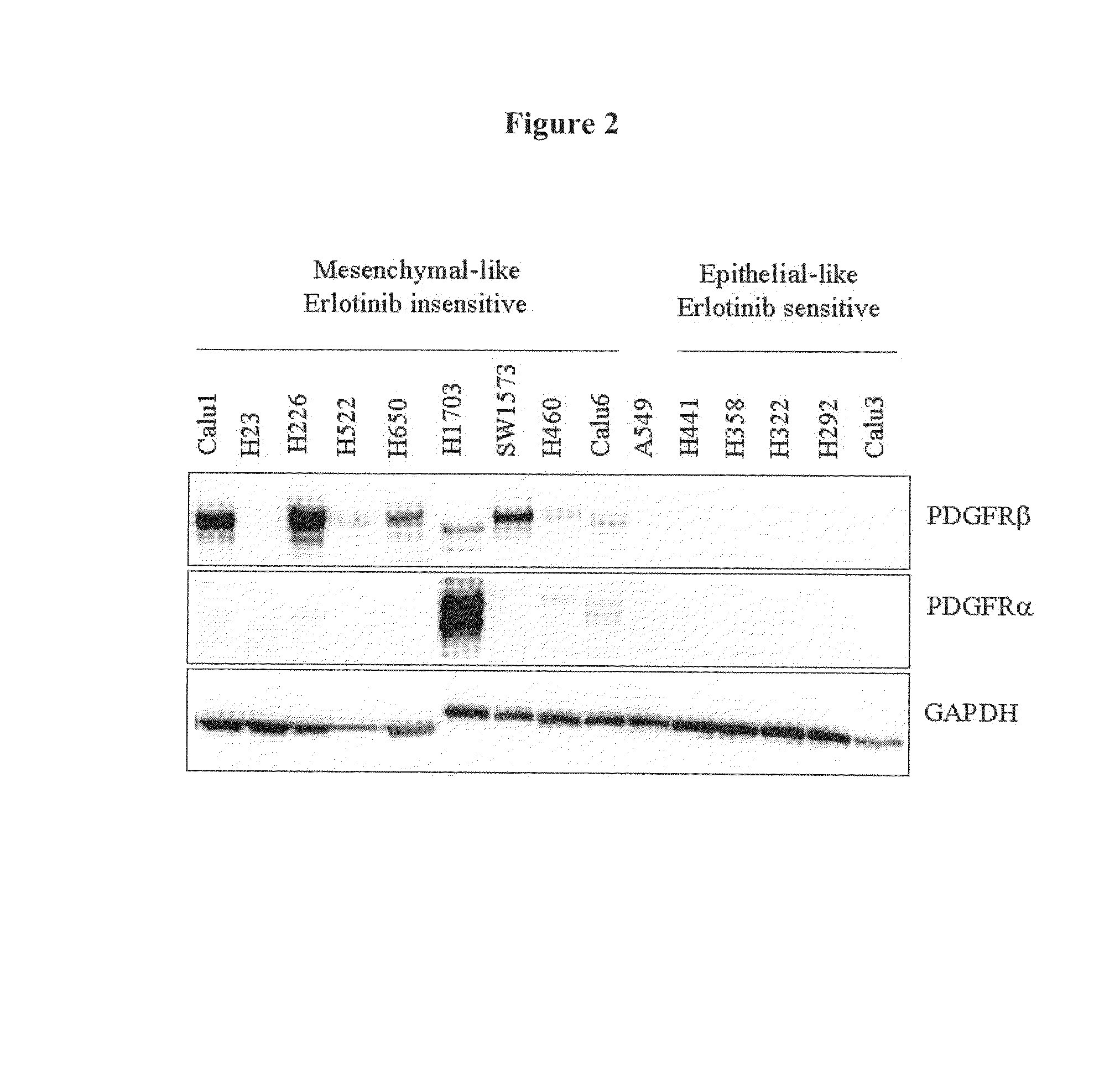
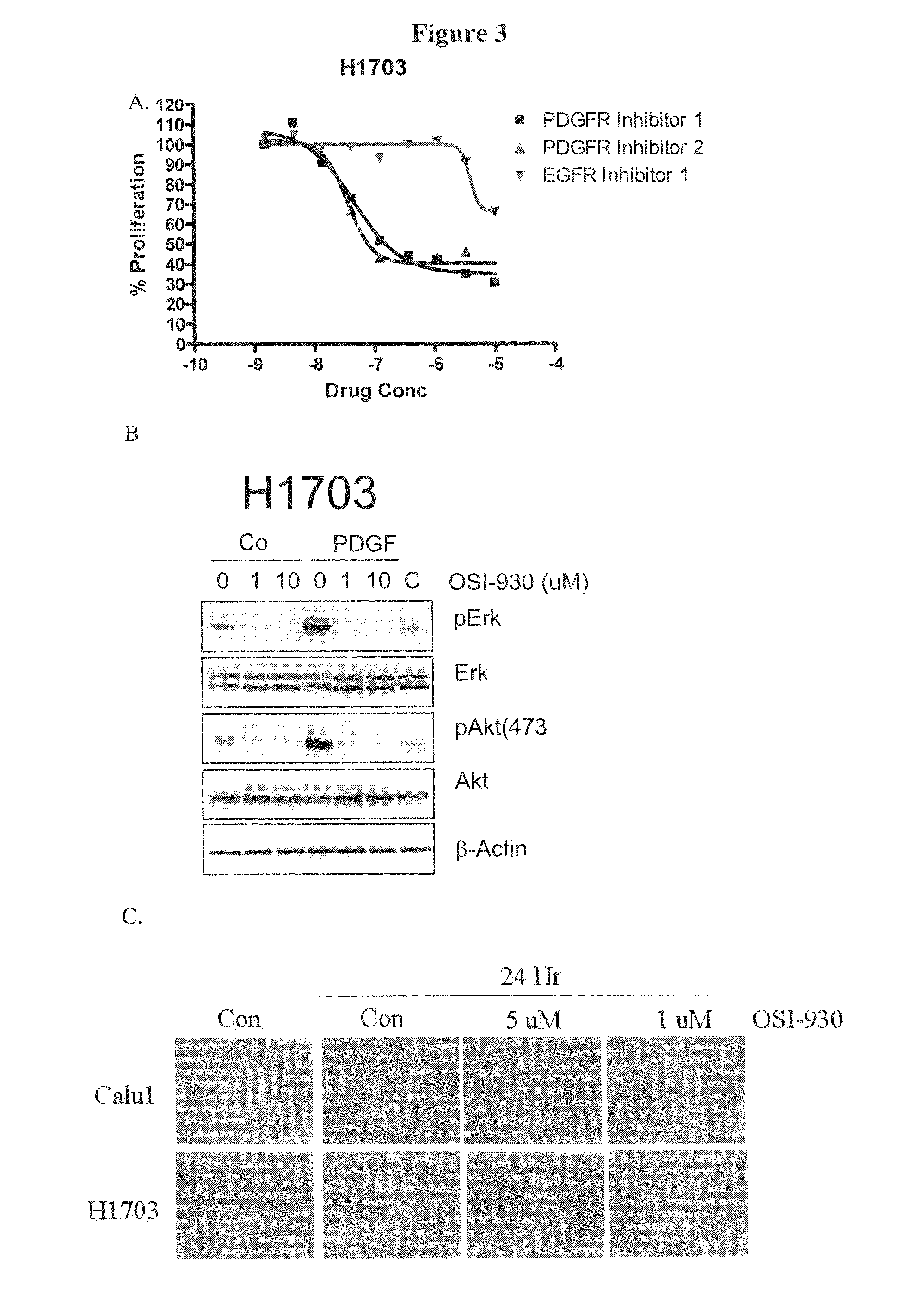
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with inhibitors of EGFR kinase, PDGFR kinase, or FGFR kinase. Based on the surprising discovery that tumors cells after having undergone an EMT, while being mesenchymal-like, still express characteristics of both epithelial and mesenchymal cells, and that such cells have altered sensitivity to inhibition by receptor protein-tyrosine kinase inhibitors, in that they have become relatively insensitive to EGFR kinase inhibitors, but have frequently acquired sensitivity to inhibitors of other receptor protein-tyrosine kinases such as PDGFR or FGFR, methods have been devised for determining levels of specific epithelial and mesenchymal biomarkers that identify such “hybrid” tumor cells (e.g. determination of co-expression of vimentin and epithelial keratins), and thus predict the tumor's likely sensitivity to inhibitors of EGFR kinase, PDGFR kinase, or FGFR kinase. Improved methods for treating cancer patients with EGFR, PDGFR or FGFR kinase inhibitors that incorporate such methodology are also provided.
Owner:OSI PHARMA LLC
Use of emt gene signatures in cancer drug discovery, diagnostics, and treatment
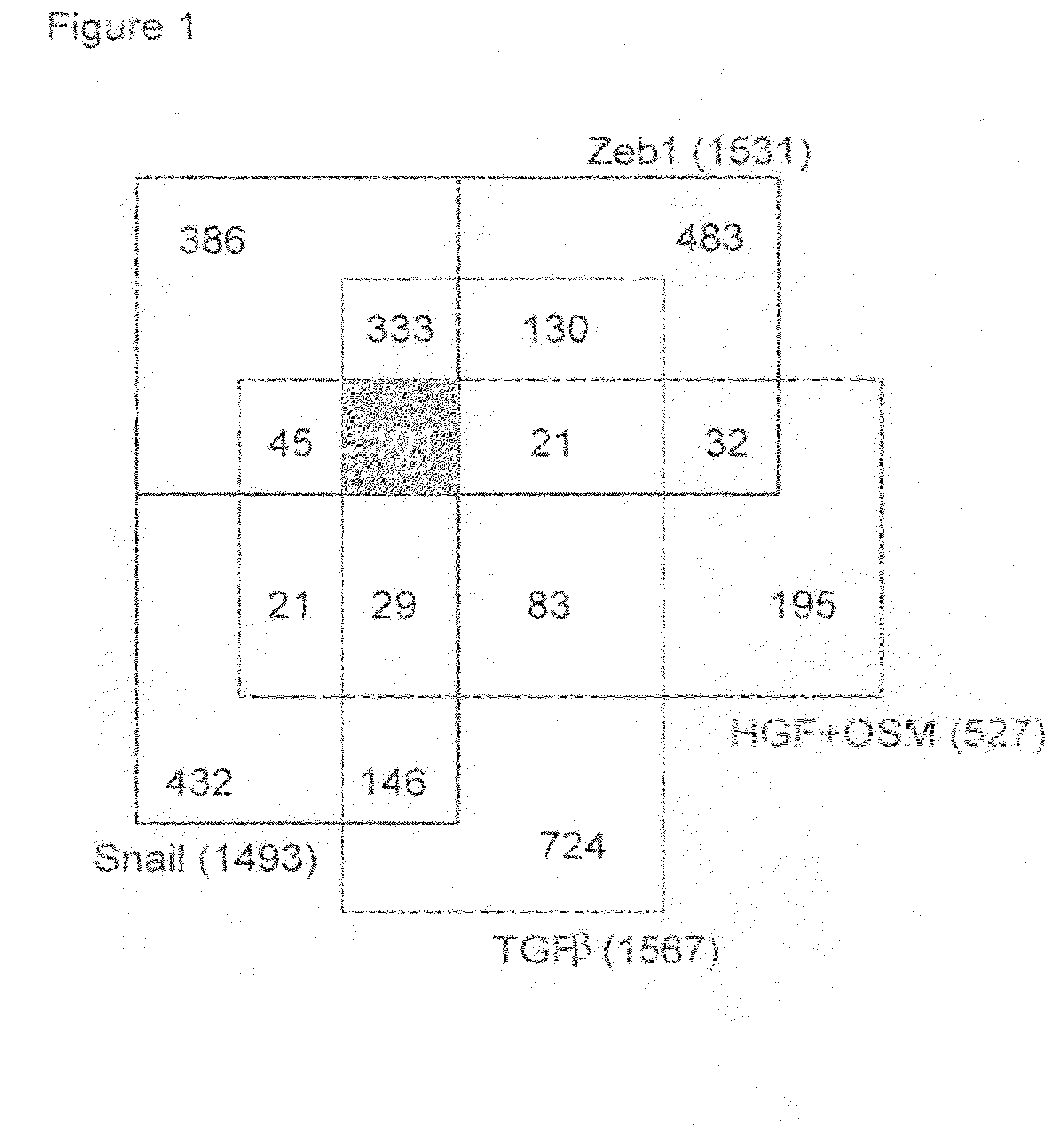
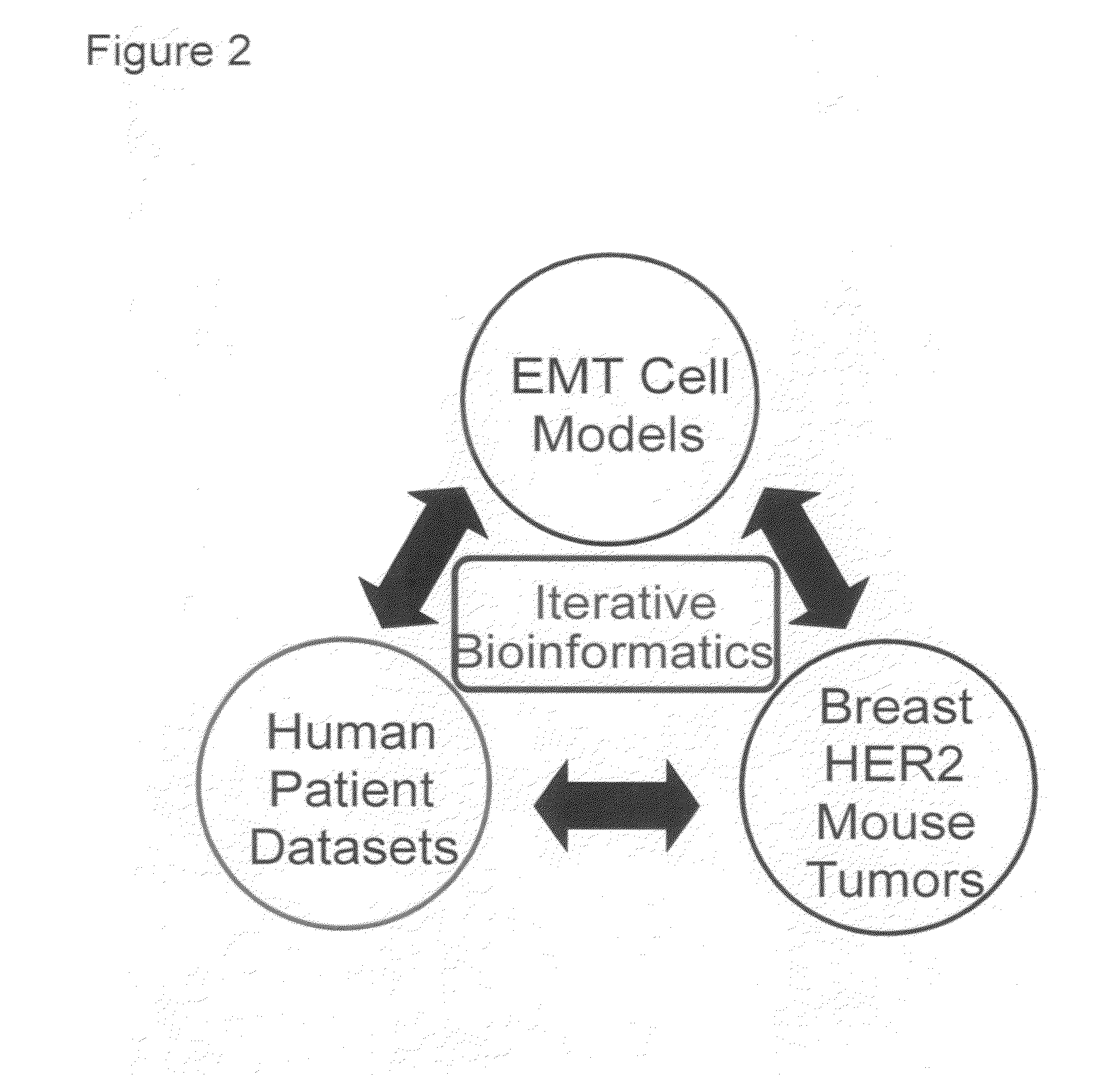
The present invention provides diagnostic methods for assessing the EMT status of tumor cells, and for predicting the effectiveness of treatment of a cancer patient with an EGFR or IGF-1R kinase inhibitor, utilizing an EMT gene signature index score. The present invention further provides methods for treating patients with cancer that incorporate these methods.
Owner:OSI PHARMA LLC
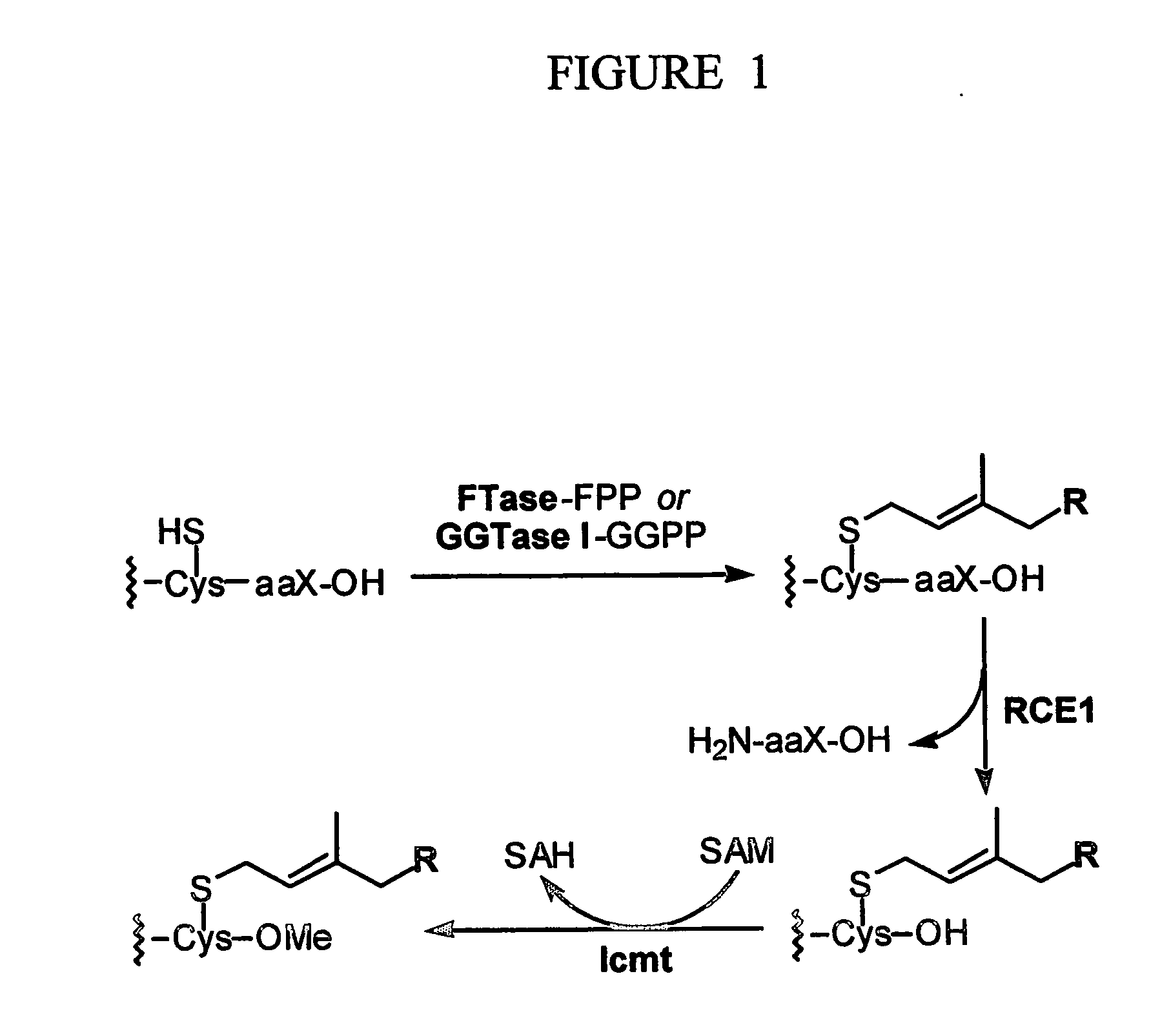
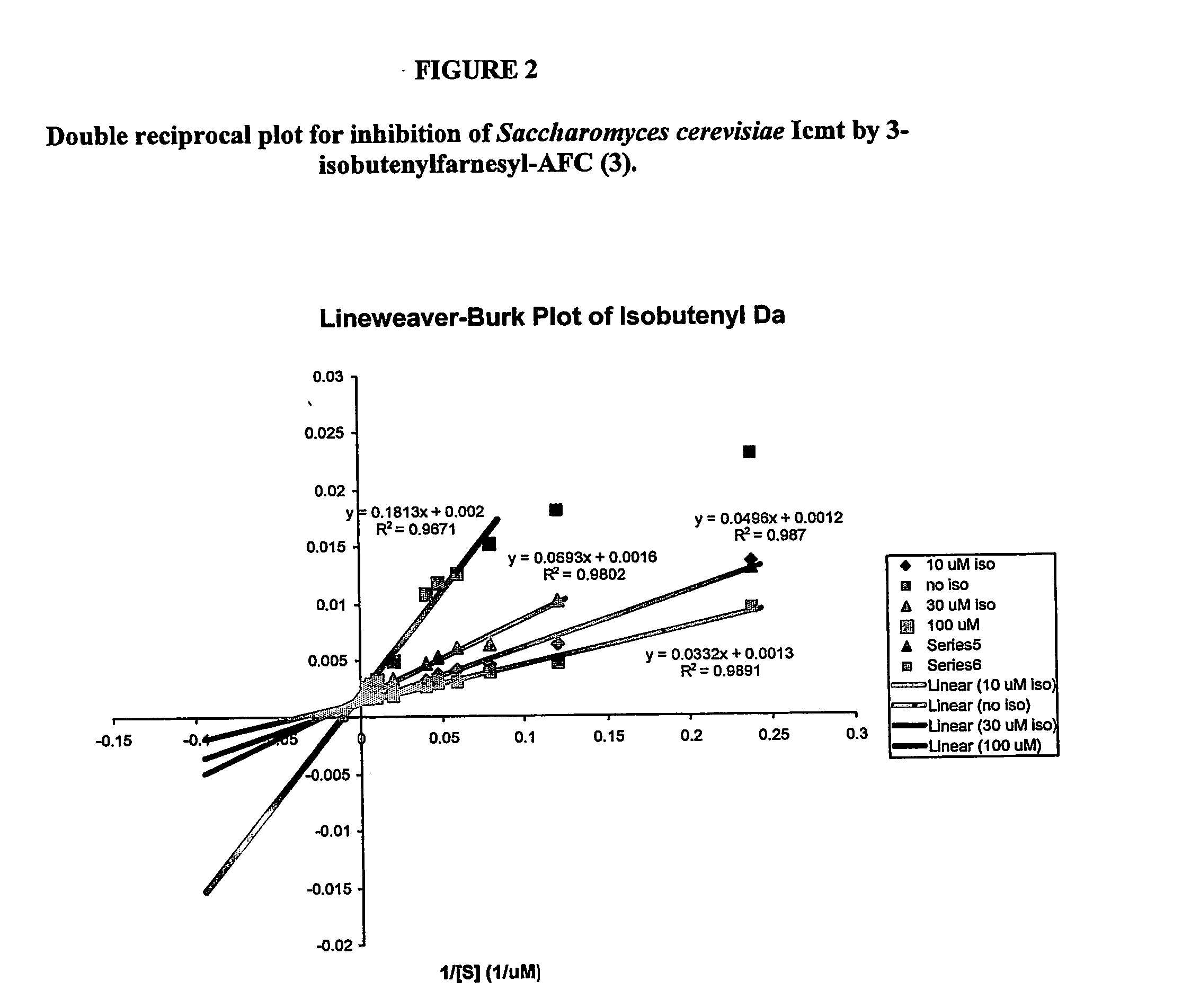
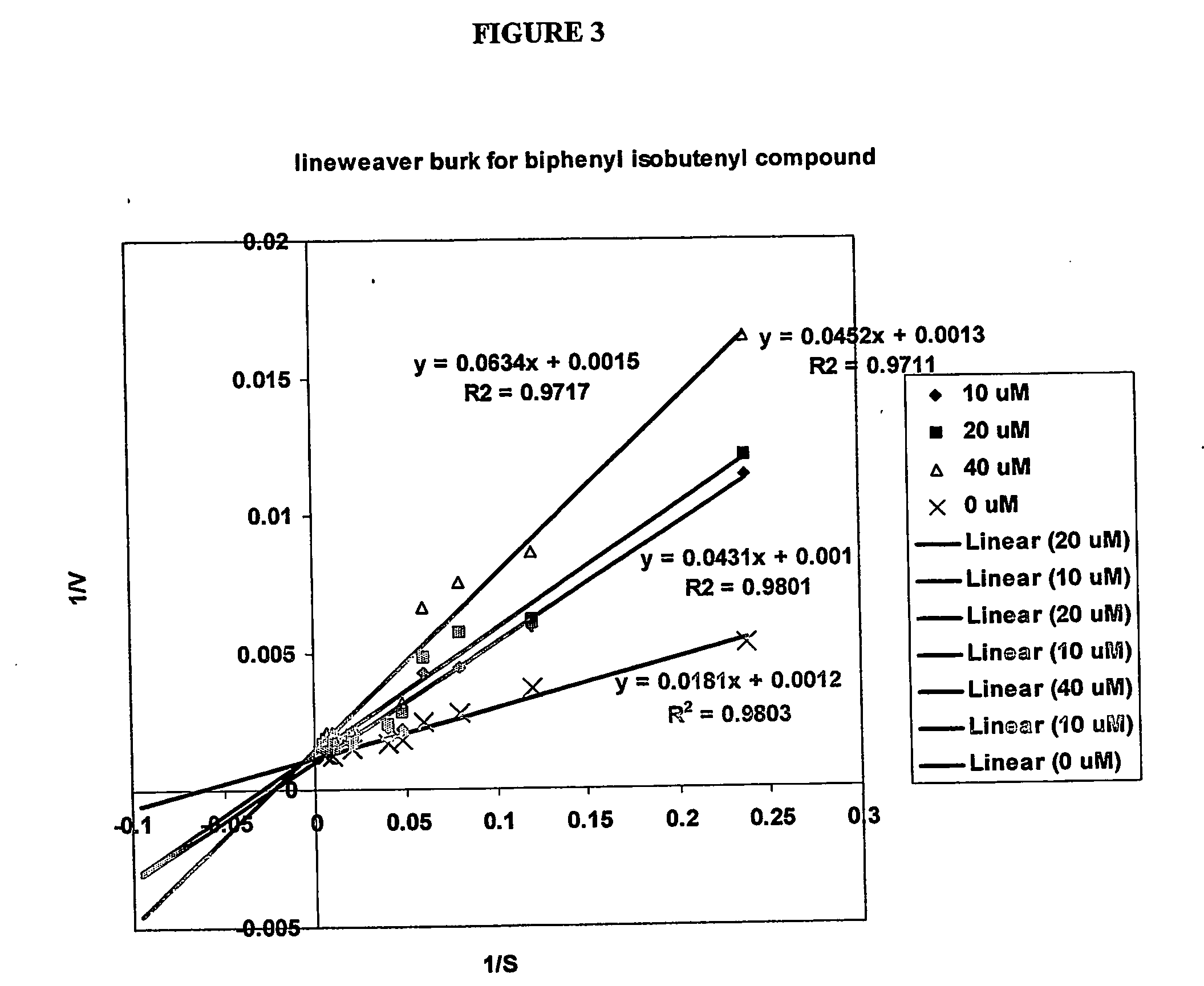
Compounds and methods for use in treating neoplasia and cancer based upon inhibitors of isoprenylcysteine methyltransferase
The present invention relates to a novel method for the treatment of neoplasia, including cancer and other diseases and conditions in humans and mammals. More particularly, in preferred aspects, the present invention provides a method for the use of prenylcysteine analogs for the treatment of neoplasia, hyperproliferative cell growth including psoriasis, restenosis following cardiovascular surgery, hyperplasia, including renal hyperplasia, chronic inflammatory diseases including rheumatoid and osteoarthritis, among others.
Owner:PURDUE RES FOUND INC
METHODS FOR TREATING CANCER USING 17alpha-HYDROXYLASE/C17,20-LYASE INHIBITORS
InactiveUS20090124587A1Reducing and avoiding progressReduced plasma concentrationOrganic active ingredientsAntineoplastic agentsMetaboliteRegimen
Owner:AUERBACH ALAN H +1
Pancreatic cancer treatment using Na+/K+ ATPase inhibitors
InactiveUS20070105790A1Patient compliance is goodKept lowBiocideCarbohydrate active ingredientsATPaseAglycone
The reagent, pharmaceutical formulation, kit, and methods of the invention provides a new approach for treating pancreatic cancers. The invention provides the use of Na+e / K+-ATPase inhibitors, such as cardiac glycosides (e.g. ouabain and proscillaridin, etc.), either alone or in combination with other standard therapeutic agents (chemo- or radio-therapies, etc.) for treating pancreatic cancers. The subject Na+ / K+-ATPase inhibitors, such as cardiac glycosides, including bufadieneolides or their corresponding aglycones (e.g., proscillaridin, scillaren, and scillarenin, etc.), especially in oral formulations and / or solid dosage forms containing more than 1 mg of active ingredients.
Owner:BIONAUT PHARMA
Biological markers predictive of Anti-cancer response to epidermal growth factor receptor kinase inhibitors
InactiveUS20120142028A1Long survival progression free survivalEffectiveness of treatmentDisease diagnosisAntineoplastic agentsEpidermal Growth Factor Receptor KinaseErlotinib
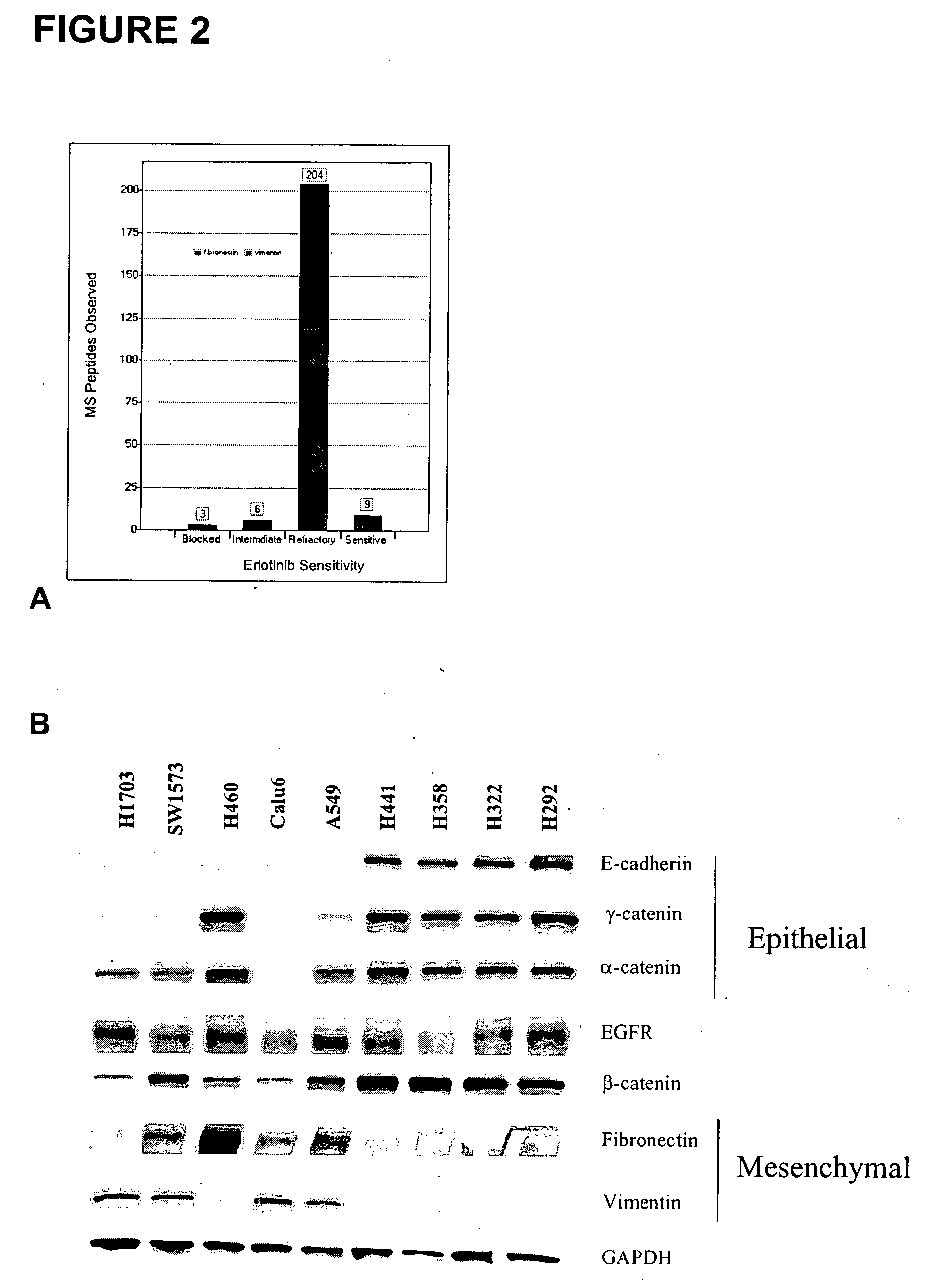
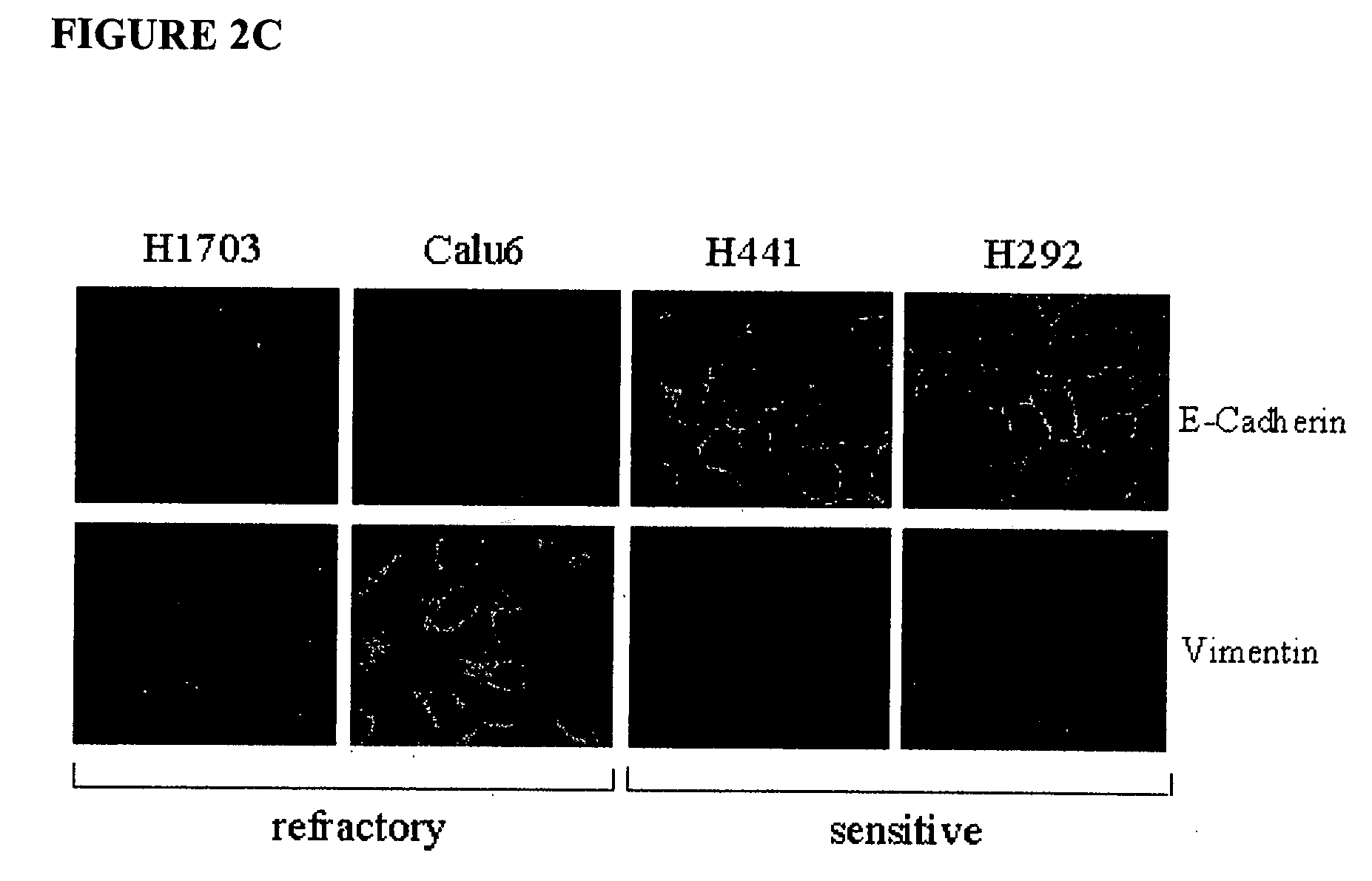
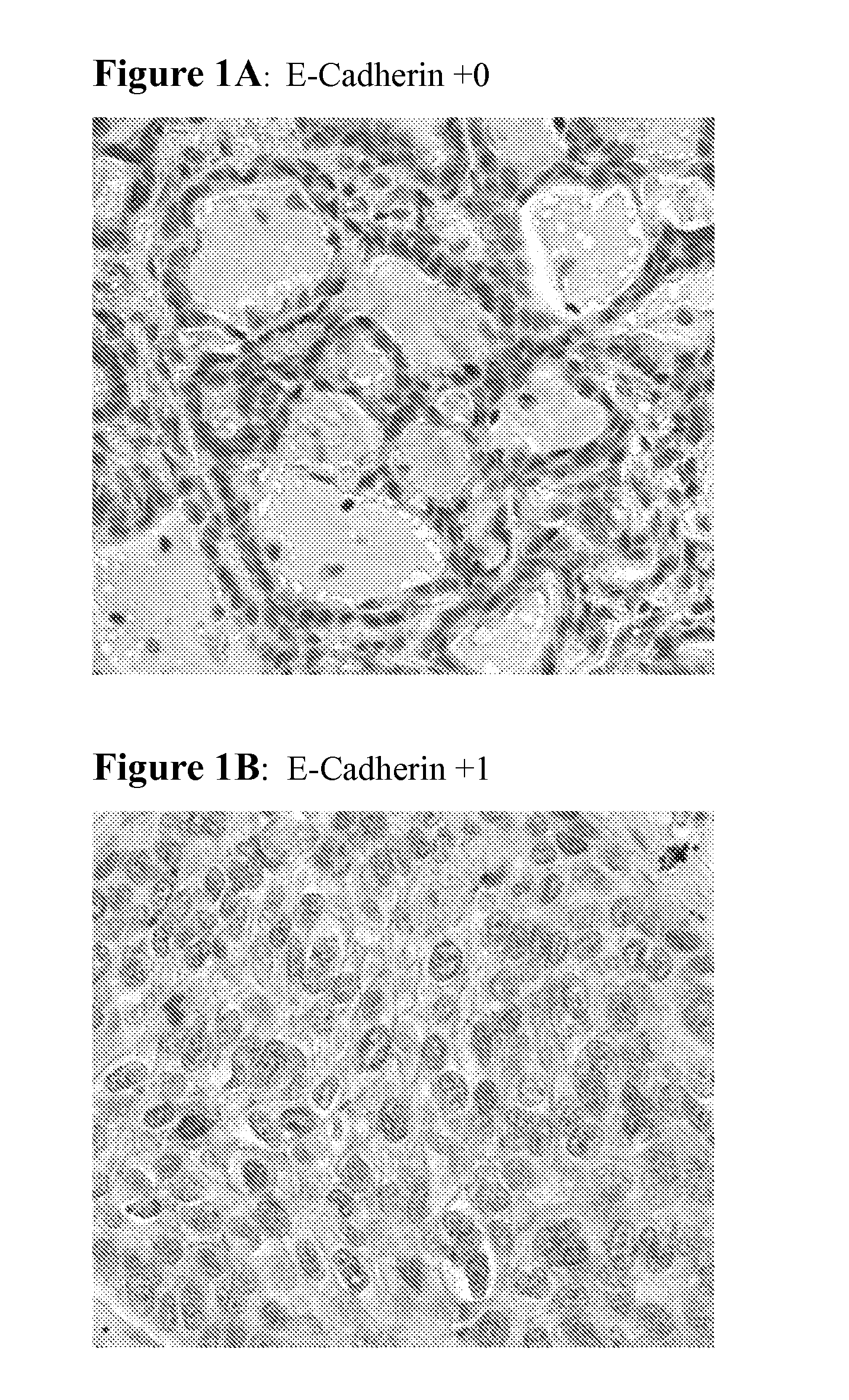
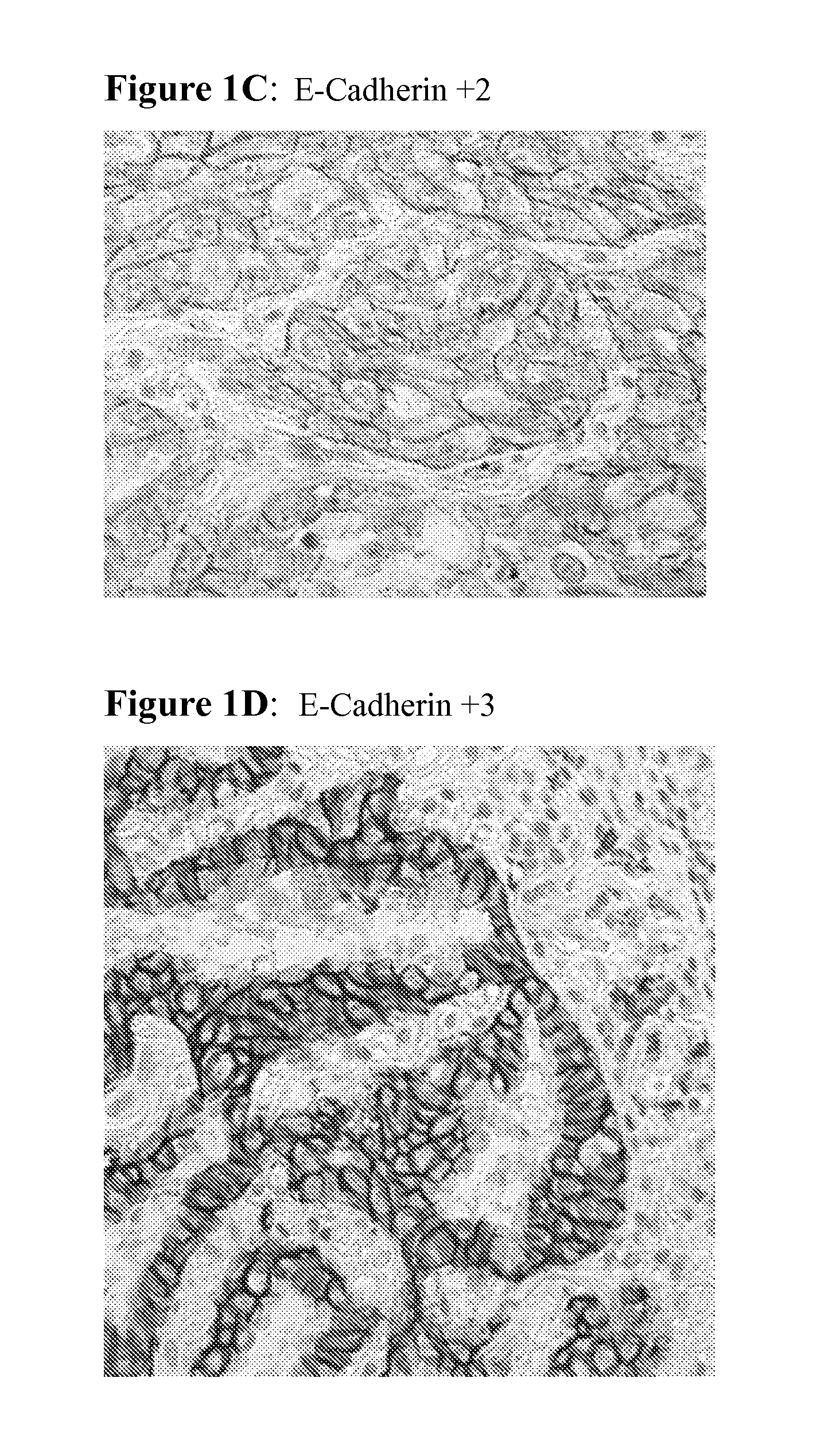
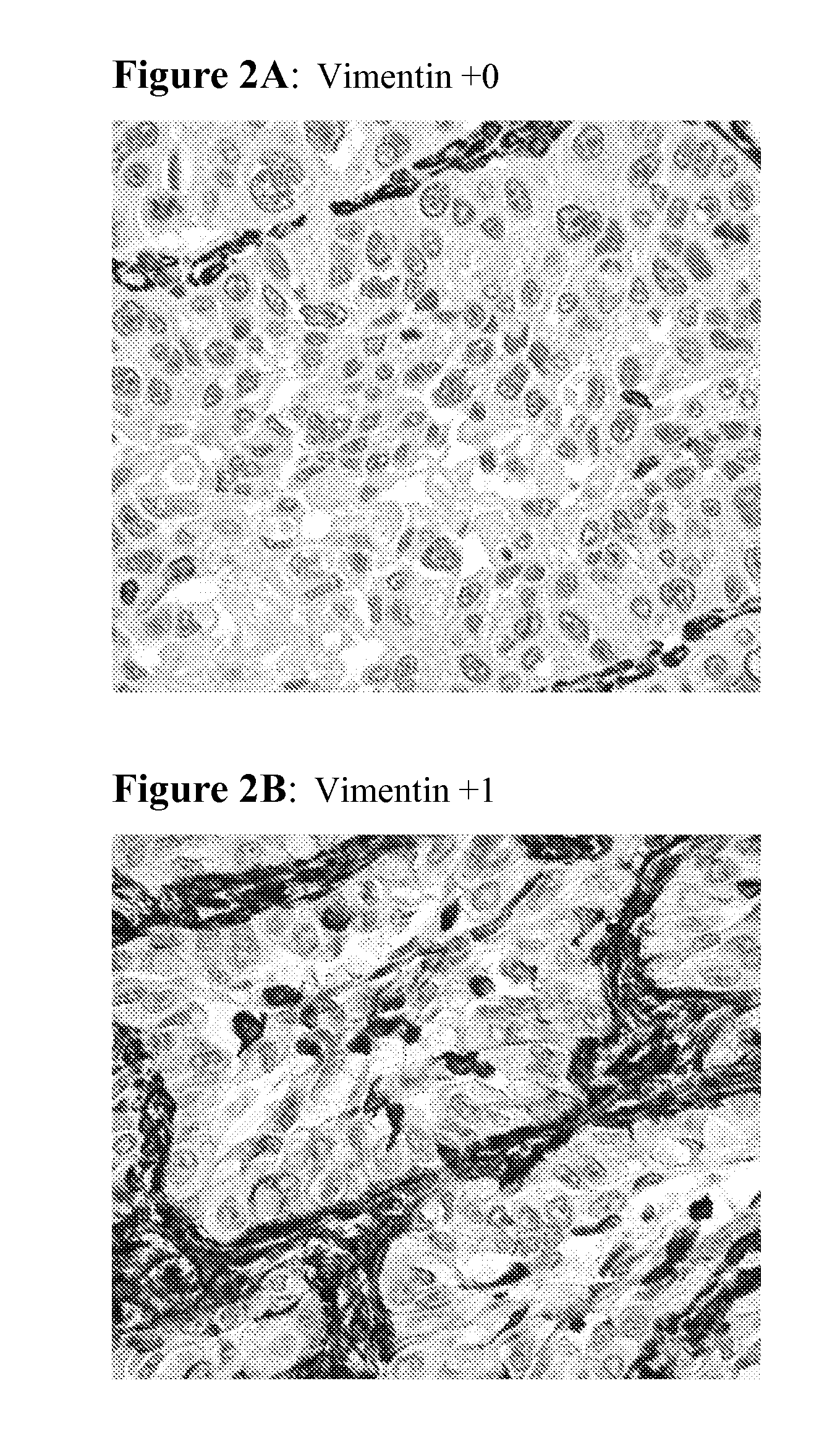
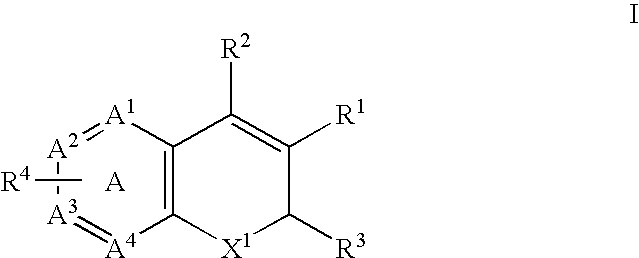
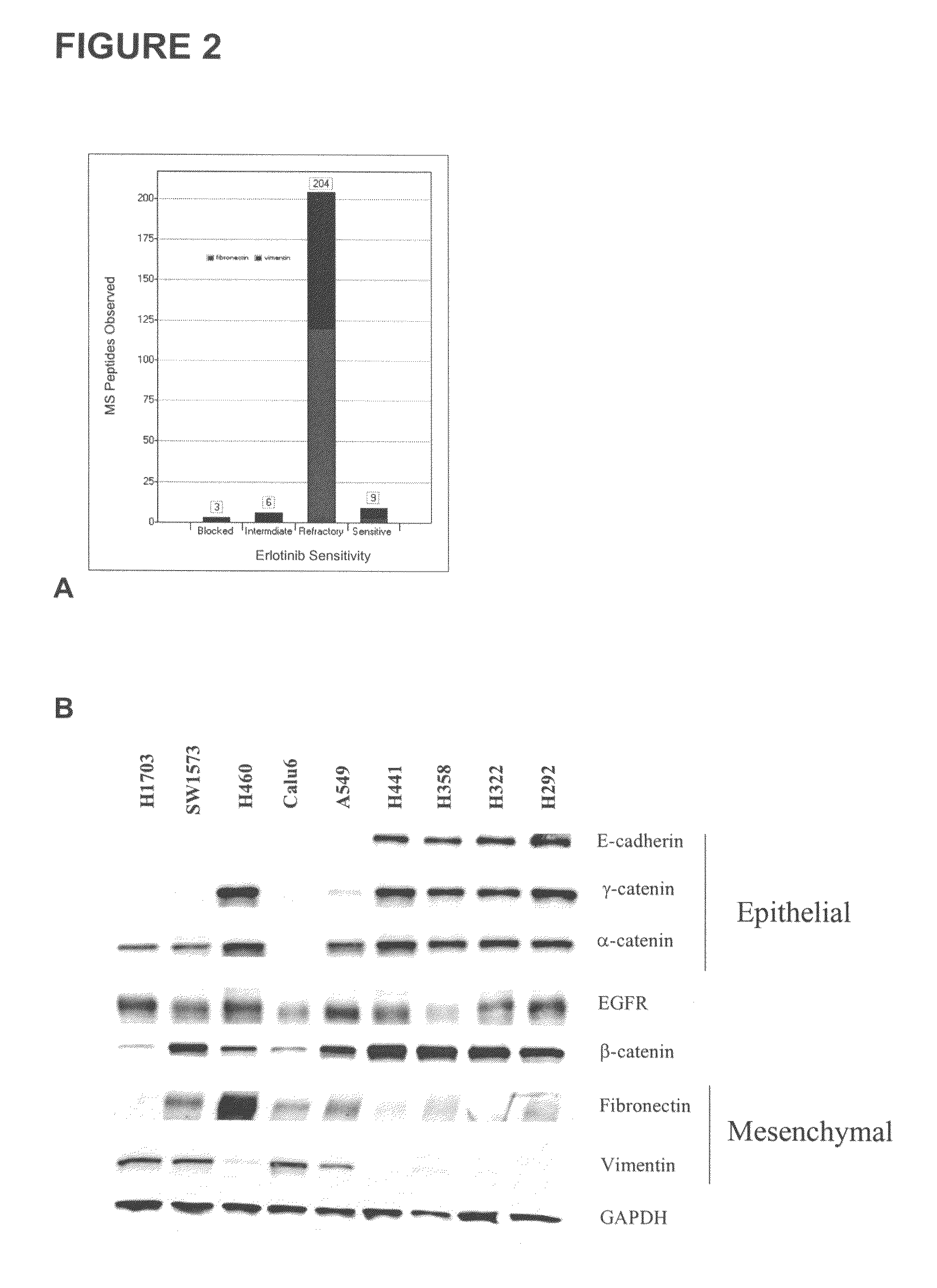
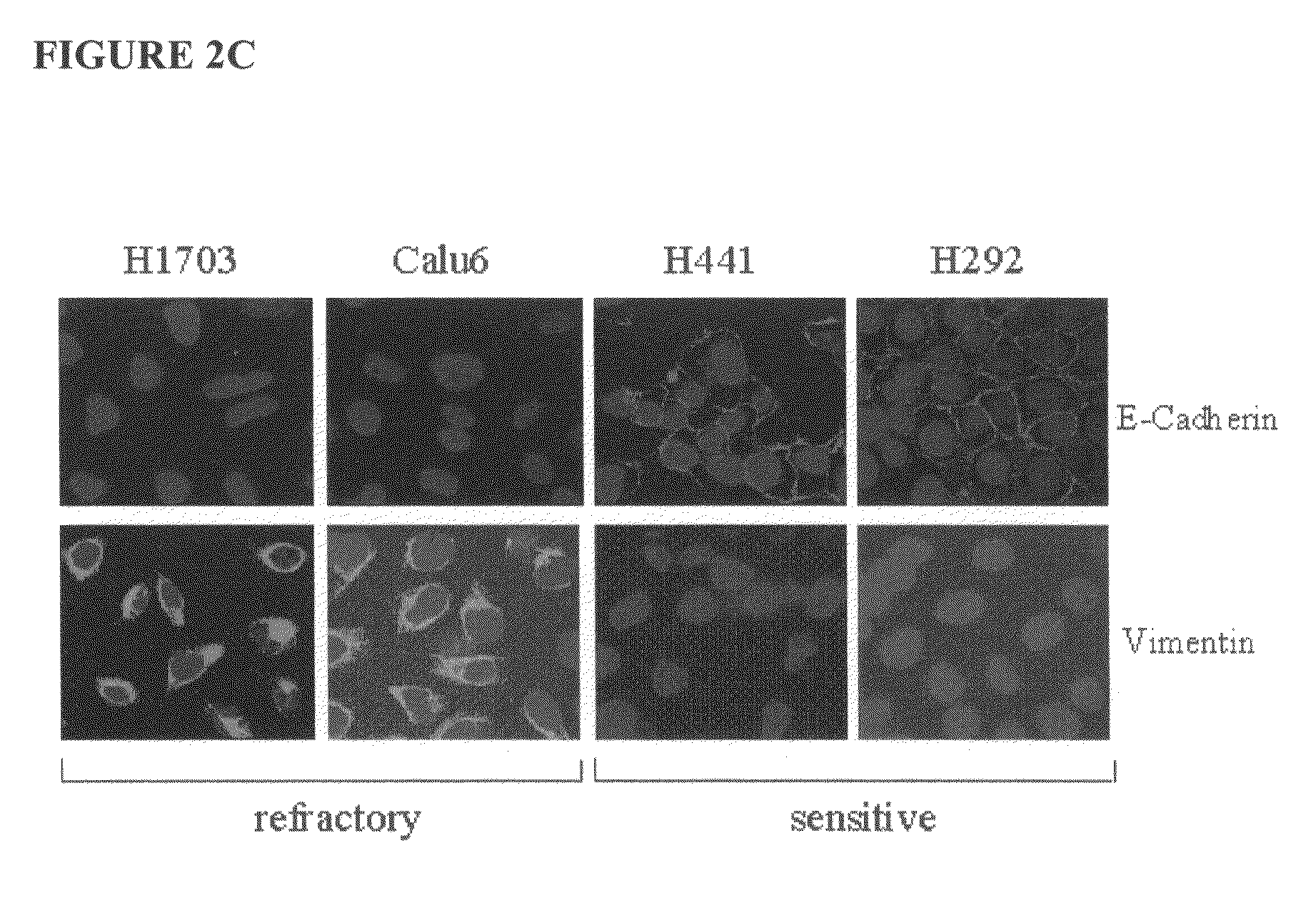
The present invention provides diagnostic methods for predicting the effectiveness of treatment of a cancer patient with an EGFR kinase inhibitor. These methods are based on the surprising discovery that the effectiveness of treatment with an EGFR kinase inhibitor is predicted by whether a patient's tumor cells express a high or a low level of the biomarkers vimentin and E-cadherin, such that patients whose tumors express a high level of at least one of the biomarkers vimentin and E-cadherin have a longer overall survival and progression free survival than patients whose tumors express a low level of both vimentin and E-cadherin. The present invention further provides a method for treating tumors or tumor metastases in a patient, comprising the steps of diagnosing a patient's likely responsiveness to an EGFR kinase inhibitor by assessing whether tumor cells express a high level of at least one of the biomarkers vimentin and E-cadherin, and administering to said patient a therapeutically effective amount of an EGFR kinase inhibitor (e.g. erlotinib), particularly when effectiveness of the inhibitor is predicted.
Owner:OSI PHARMA LLC
Treatment or prevention of vascular disorders with Cox-2 inhibitors in combination with cyclic AMP-specific phosphodiesterase inhibitors
InactiveUS20050187278A1Efficacious and safe and easy to administerBiocideAmide active ingredientsCOX-2 inhibitorPhosphodiesterase inhibitor
A method is described for the prevention and / or treatment of vascular disorders and vascular disorder-related complications in a subject, the method comprising administering to the subject a Cox-2 inhibitor in combination with a cAMP-specific PDE inhibitor. Also described are therapeutic and pharmaceutical compositions and kits that are useful in the present invention.
Owner:PHARMACIA CORP
Treatment of refractory cancers using Na+/K+-ATPase inhibitors
InactiveUS20080027010A1Improve stress responseGood tumor growthBiocideOrganic active ingredientsATPaseAglycone
The reagent, pharmaceutical formulation, kit, and methods of the invention provides a new approach to treat refractory cancers using Na+ / K+-ATPase inhibitors, such as cardiac glycosides, including bufadienolides or their corresponding aglycones (e.g., proscillaridin, scillaren, and scillarenin, etc.), especially in oral formulations and / or solid dosage forms containing more than 1 mg of active ingredients.
Owner:BIONAUT PHARMA
Biological markers predictive of anti-cancer response to epidermal growth factor receptor kinase inhibitors
ActiveUS8093011B2Organic active ingredientsDisease diagnosisEpidermal Growth Factor Receptor KinaseCell growth
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with an EGFR kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an EGFR kinase inhibitor, comprising assessing whether the tumor cell has undergone an epithelial to mesenchymal transition (EMT), by determining the expression level of epithelial and / or mesenchymal biomarkers, wherein tumor cells that have undergone an EMT are substantially less sensitive to inhibition by EGFR kinase inhibitors. Improved methods for treating cancer patients with EGFR kinase inhibitors that incorporate the above methodology are also provided. Additionally, methods are provided for the identification of new biomarkers that are predictive of responsiveness of tumors to EGFR kinase inhibitors. Furthermore, methods for the identification of agents that restore the sensitivity of tumor cells that have undergone EMT to inhibition by EGFR kinase inhibitors are also provided.
Owner:OSI PHARMA INC
Biological markers predictive of Anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors
The present invention provides diagnostic methods for predicting the effectiveness of treatment of a cancer patient with an IGF-1R kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an IGF-1R kinase inhibitor, comprising assessing whether the tumor cell expresses certain sensitivity or resistance biomarkers, or genomic classifiers. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate this methodology are also provided.
Owner:UNIV OF COLORADO THE REGENTS OF
Biological markers predictive of Anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors
InactiveUS20110275644A1Increase valueBiocideMicrobiological testing/measurementImproved methodBiomarker (petroleum)
The present invention provides diagnostic methods for predicting the effectiveness of treatment of a cancer patient with an IGF-1R kinase inhibitor that inhibits both IGF-1R and IR kinases. Methods are provided for identifying patients with cancer who are likely to benefit from treatment with an IGF-1R kinase inhibitor that inhibits both IGF-1R and IR kinases. Methods are also provided for identifying patients with cancer who are likely to benefit from treatment with an IGF-1R kinase inhibitor that inhibits both IGF-1R and IR kinases, but who would likely not respond to therapy with an anti-IGF-1R antibody. Methods are also provided for identifying patients with cancer who are more likely to benefit from treatment with anti-IGF-1R antibody. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate these methods are also provided.
Owner:OSI PHARMA INC
Treating Learning Deficits With Inhibitors of Hmg CoA Reductase
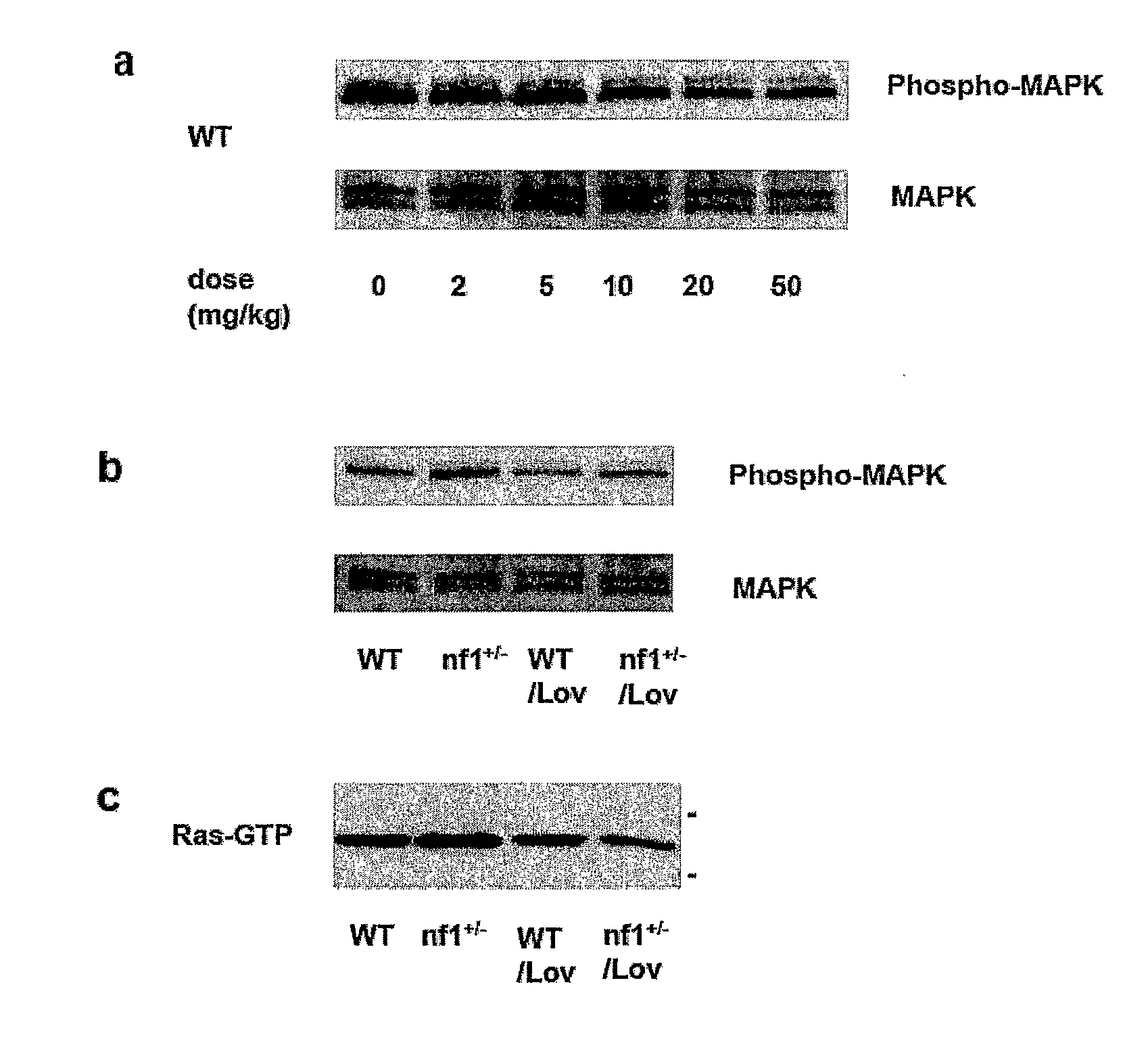
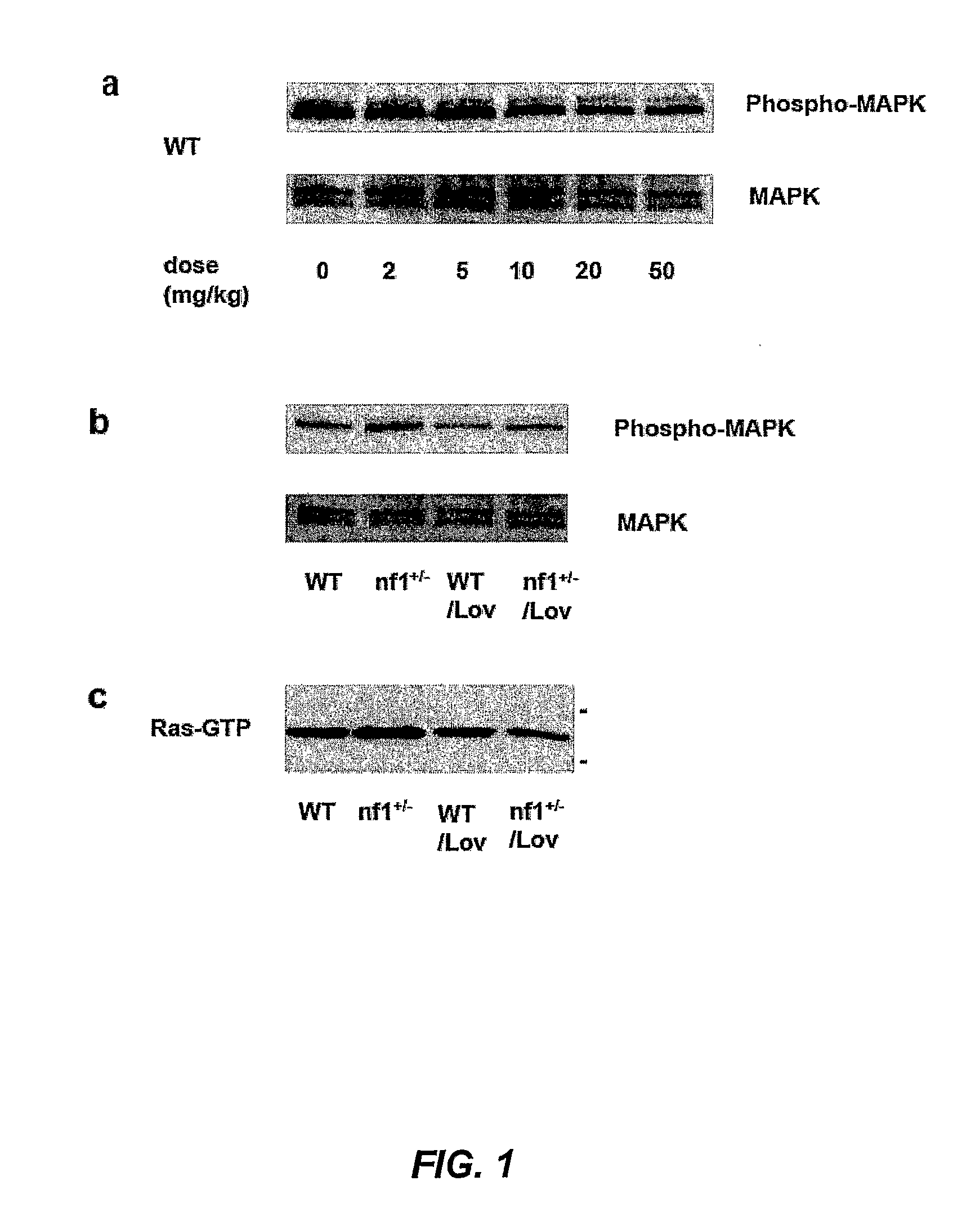
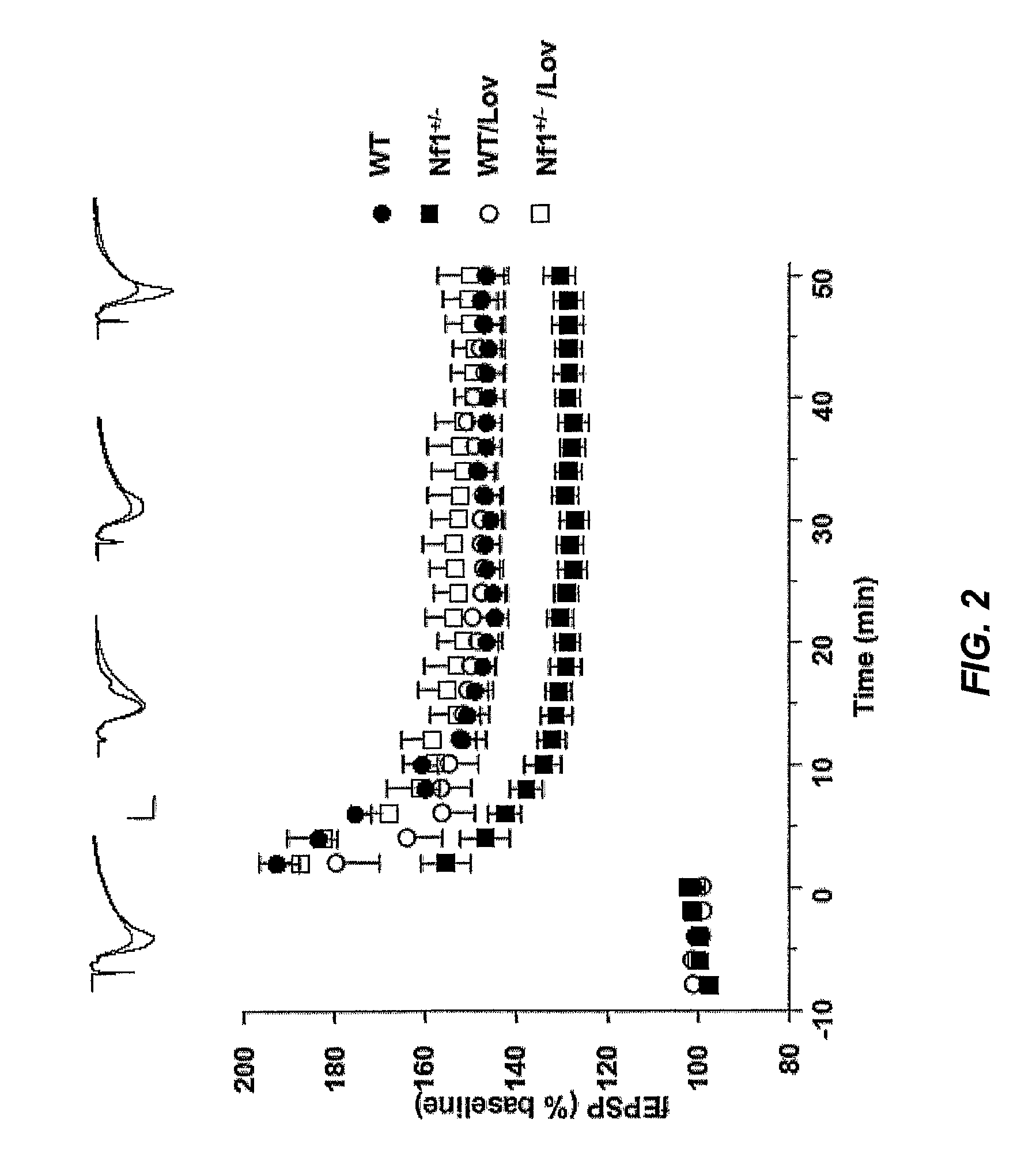
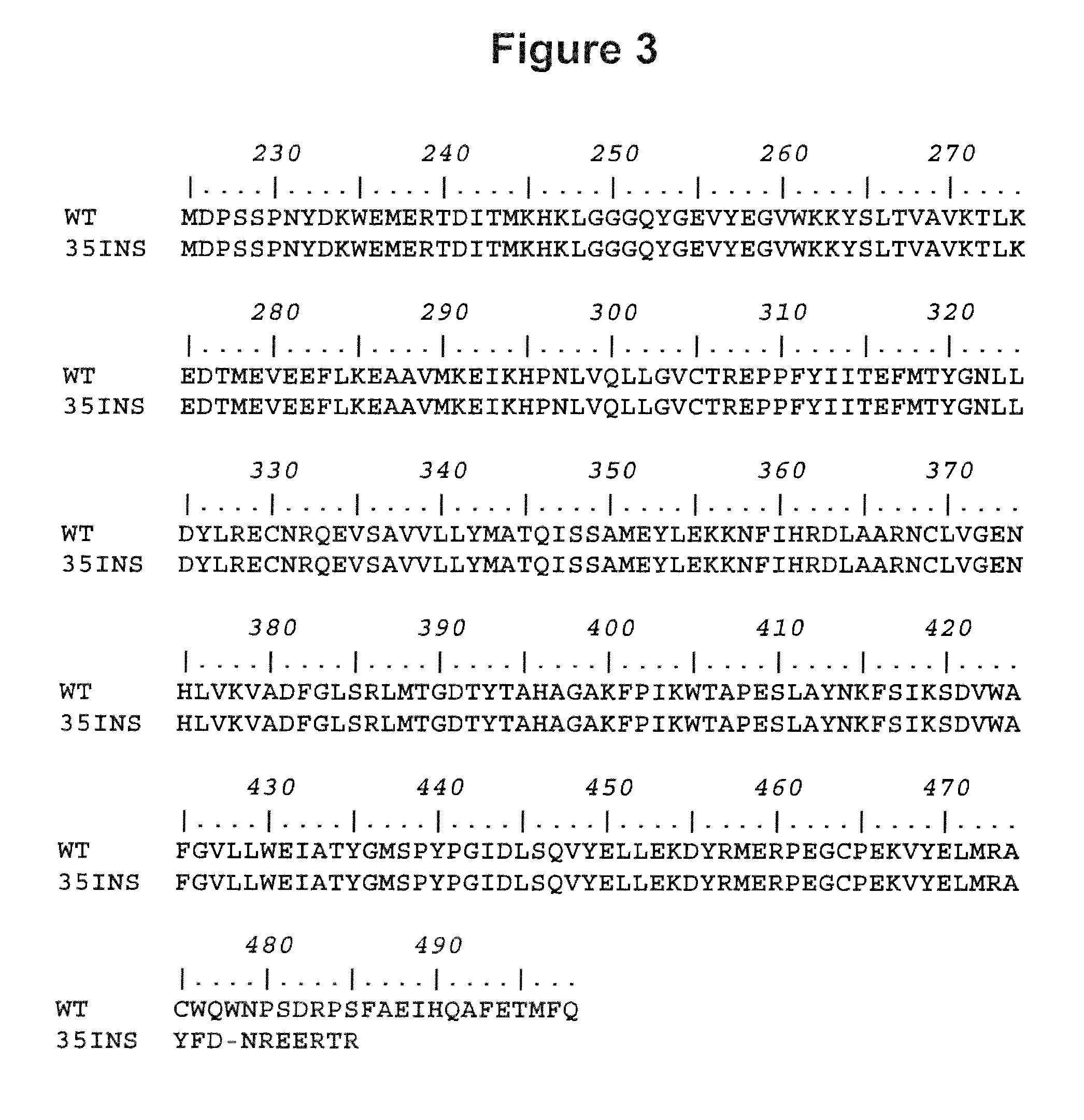
The disclosure provides methods of treating cognitive disorders by administering a HMG CoA reductase inhibitor. Cognitive deficits treatable with the inhibitor compound include those associated with Angelman Syndrome, Neurofibromatosis-1, certain forms of X-linked mental retardation, tuberous sclerosis, Down Syndrome, autism, and attention deficit / hyperactivity disorder.
Owner:RGT UNIV OF CALIFORNIA
Bcr-abl variants
ActiveUS20100113470A1Reduced growth rateReduced viabilityBiocideOrganic active ingredientsBcr abl kinaseGene product
A splice variant of bcr-abl mRNA that produces BCR-ABL protein with a truncated C-terminus and its role in resistance to treatment with kinase inhibitors is disclosed. Vectors for expressing the truncated gene product are provided as well as recombinant cells that express the truncated gene product from a cDNA construct. Also provided are methods compositions and kits for detecting the BCR-ABL splice variant. Additionally, methods for screening BCR-ABL kinase domain inhibitors which rely on the recombinant cells and methods of predicting likelihood for resistance of a CML patient with a BCR / ABL translocation respond to treatment with one or more BCR-ABL kinase inhibitors are also disclosed.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
Biological markers predictive of anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with an IGF-1R kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an IGF-1R kinase inhibitor, comprising assessing whether the tumor cell has undergone an epithelial to mesenchymal transition (EMT), by determining the expression level of epithelial and / or mesenchymal biomarkers, wherein tumor cells that have undergone an EMT are substantially less sensitive to inhibition by IGF-1R kinase inhibitors. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate the above methodology are also provided. Additionally, methods are provided for the identification of new biomarkers that are predictive of responsiveness of tumors to IGF-1R kinase inhibitors. Furthermore, methods for the identification of agents that restore the sensitivity of tumor cells that have undergone EMT to inhibition by IGF-1R kinase inhibitors are also provided. pErk, HER3 and pHER are also demonstrated to be effective biomarkers for predicting sensitivity of tumor cells to IGF-1R kinase inhibitors.
Owner:OSI PHARMA INC
Prostate cancer treatment with glycogen synthase kinase-3beta inhibitors
InactiveUS20070196514A1Relieve painAvoid painSalicyclic acid active ingredientsBiocideAndrogenGlycogen synthase I
Owner:UNIV KANSAS MEDICAL CENT
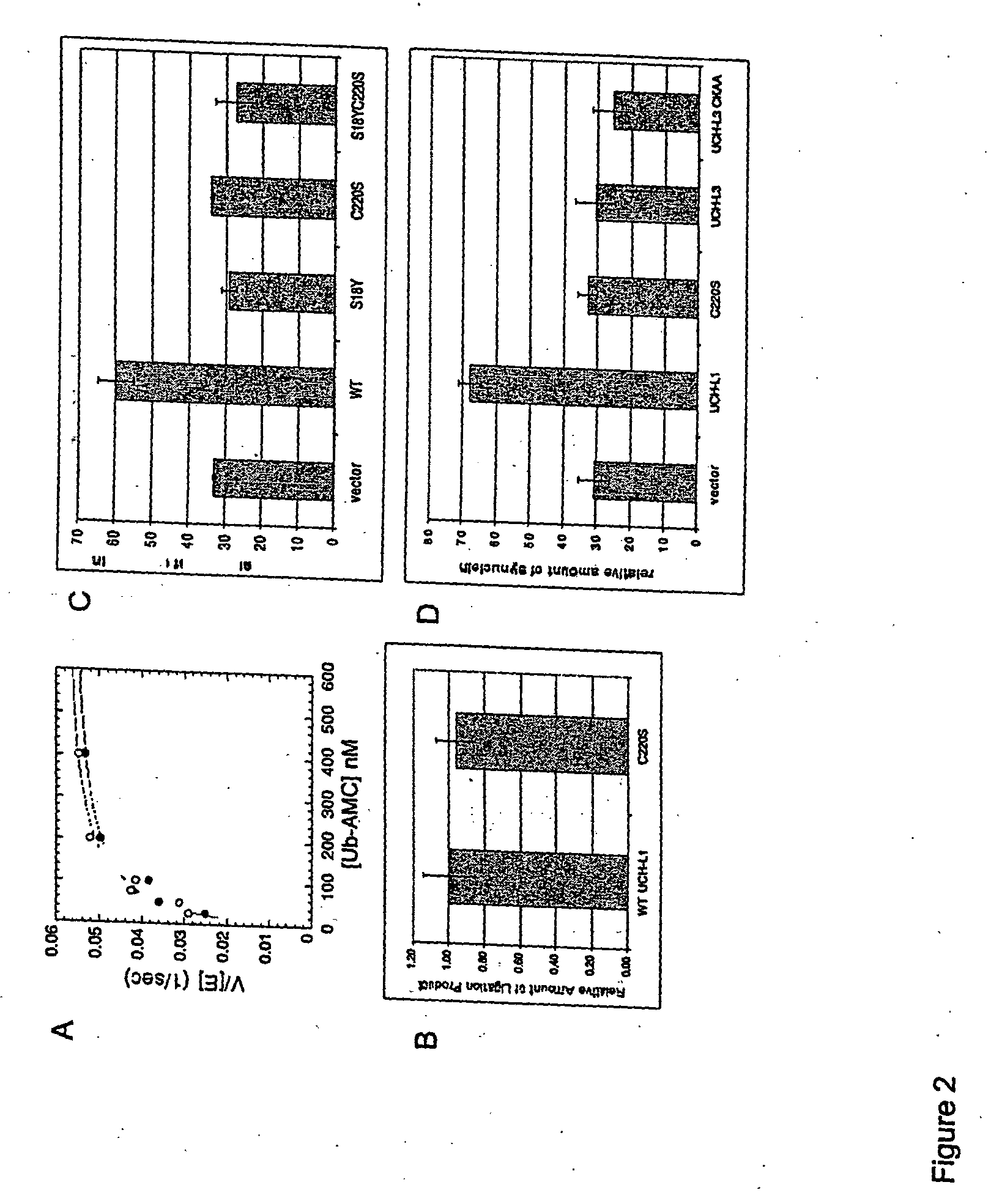
UCH-L1 expression and cancer therapy
InactiveUS20050272068A1Reduce anti-proliferative effect of UCH-LImprove the level ofMicrobiological testing/measurementMaterial analysisTransferase inhibitorCancer therapy
Methods are provided of treating cancer with one or more farnesyl transferase inhibitors. Methods are provided for identifying cancers that are particularly susceptible to treatment with one or more farneseyl transferase inhibitors by identifying cancers that express low levels of UCH-L1.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Panel of biomarkers for prediction of fti efficacy
Owner:SCHERING CORP
Biological markers predictive of anti-cancer response to kinase inhibitors
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with inhibitors of EGFR kinase, PDGFR kinase, or FGFR kinase. Based on the surprising discovery that tumors cells after having undergone an EMT, while being mesenchymal-like, still express characteristics of both epithelial and mesenchymal cells, and that such cells have altered sensitivity to inhibition by receptor protein-tyrosine kinase inhibitors, in that they have become relatively insensitive to EGFR kinase inhibitors, but have frequently acquired sensitivity to inhibitors of other receptor protein-tyrosine kinases such as PDGFR or FGFR, methods have been devised for determining levels of specific epithelial and mesenchymal biomarkers that identify such “hybrid” tumor cells (e.g. determination of co-expression of vimentin and epithelial keratins), and thus predict the tumor's likely sensitivity to inhibitors of EGFR kinase, PDGFR kinase, or FGFR kinase. Improved methods for treating cancer patients with EGFR, PDGFR or FGFR kinase inhibitors that incorporate such methodology are also provided.
Owner:OSI PHARMA LLC
Biological markers predictive of Anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors
InactiveUS20120201832A1Microbiological testing/measurementAntibody ingredientsCell growthImproved method
The present invention provides diagnostic methods for predicting the effectiveness of treatment of a cancer patient with an IGF-1R kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an IGF-1R kinase inhibitor, comprising assessing whether the tumor cell expresses certain sensitivity or resistance biomarkers, or genomic classifiers. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate this methodology are also provided.
Owner:UNIV OF COLORADO THE REGENTS OF
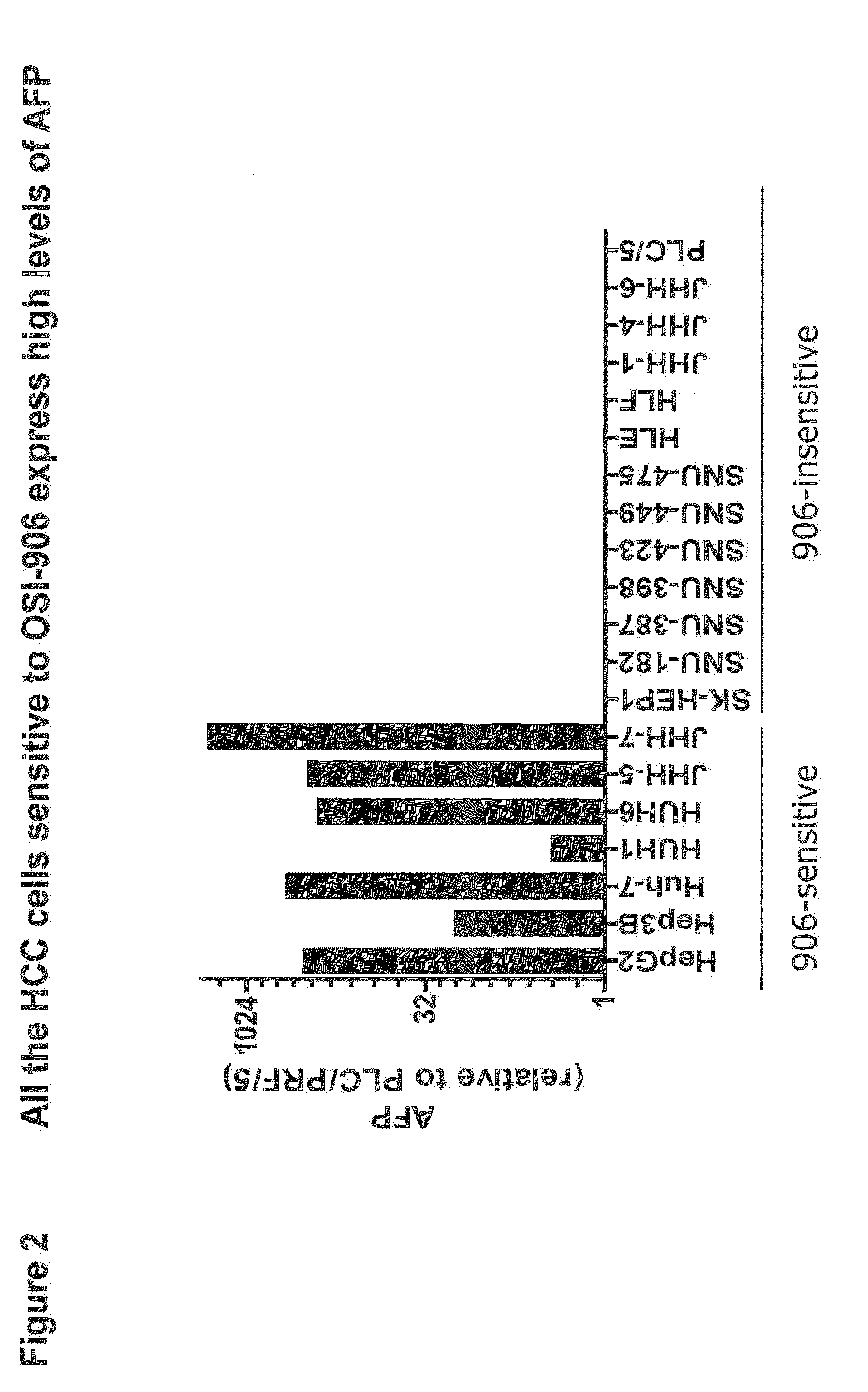
Biological markers predictive of Anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors in hepatocellular carcinoma
InactiveUS20120214830A1Improve the level ofHigh sensitivityBiocideOrganic active ingredientsAbnormal tissue growthHepatocellular carcinoma
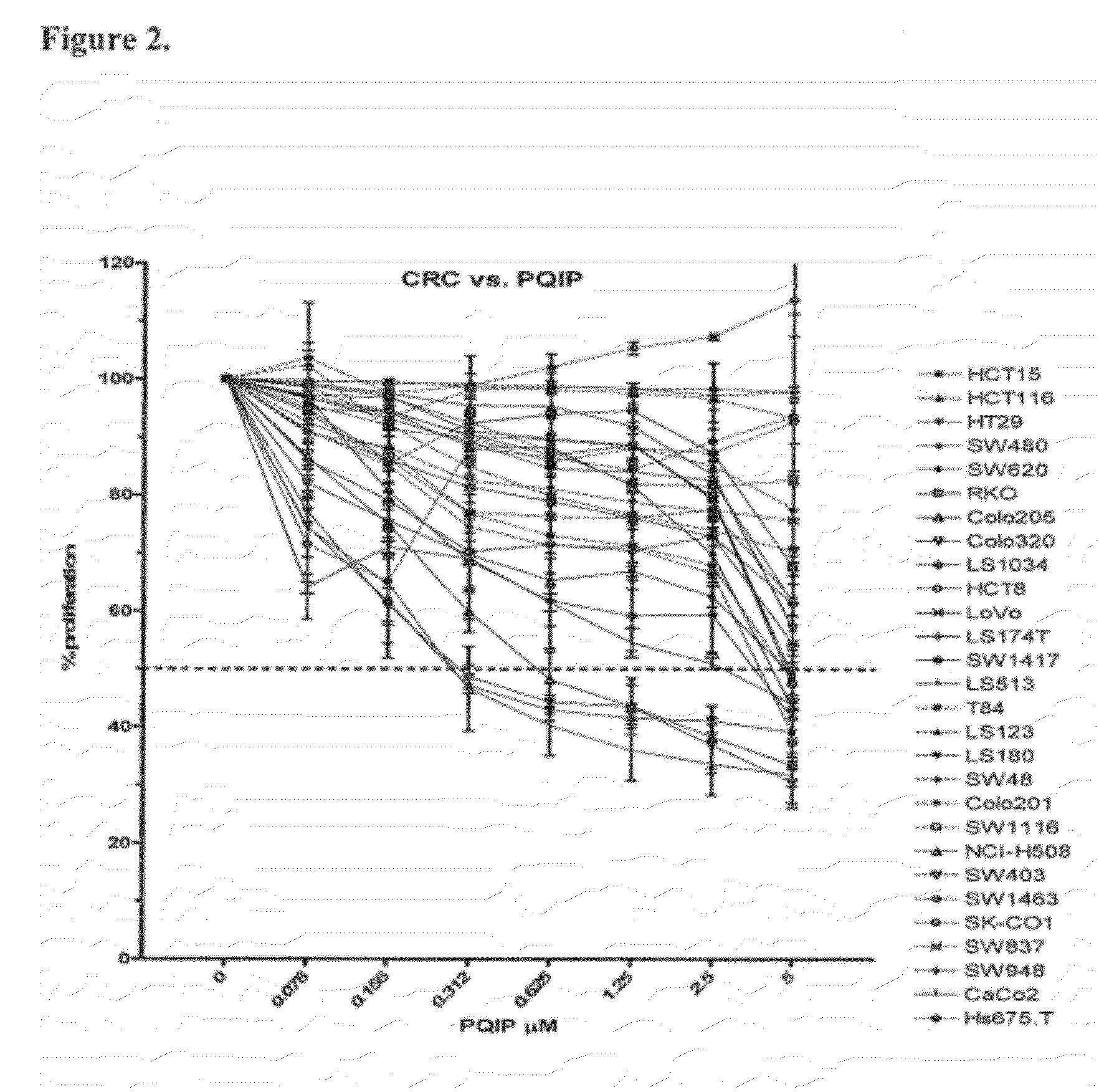
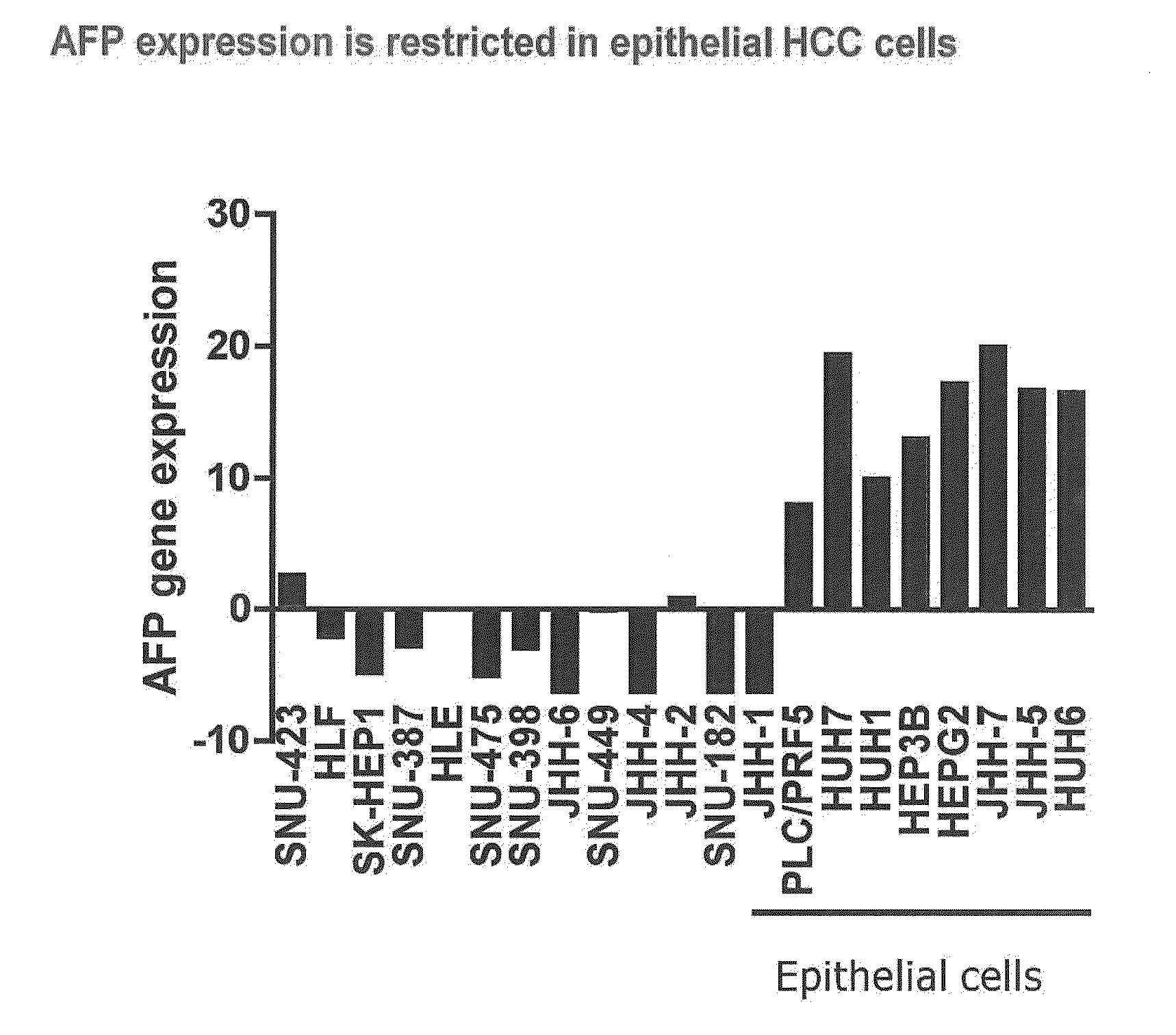
The present invention provides diagnostic methods for predicting the effectiveness of treatment of an hepatocellular carcinoma (HCC) patient with an IGF-1R kinase inhibitor by assessing whether the HCC tumor cells express a high level of AFP, or whether serum levels of AFP protein are high. The present invention also provides diagnostic methods for predicting the effectiveness of treatment of cancer patients with IGF-1R kinase inhibitors, based on a determination of the expression level of IR, IGF-2, IGFBP3 or IGFBP7 in tumor cells, or a 4-gene index calculated using the expression vales for each of these four genes, which can be used to identify tumors that will be sensitive to IGF-1R kinase inhibitors, and also those that will be insensitive. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate the above methods are also provided.
Owner:OSI PHARMA LLC
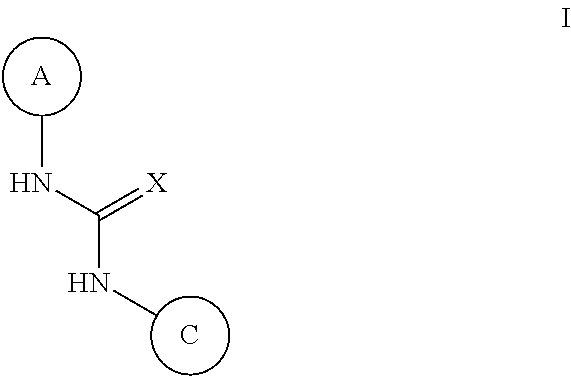
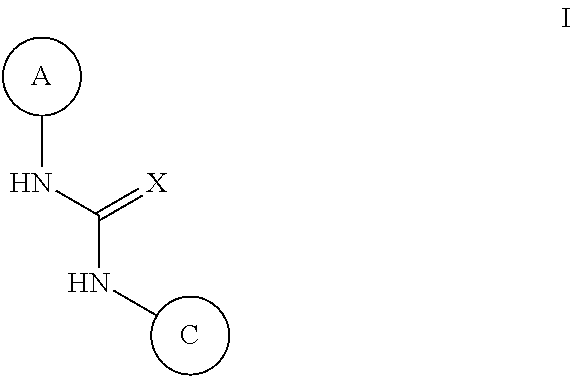
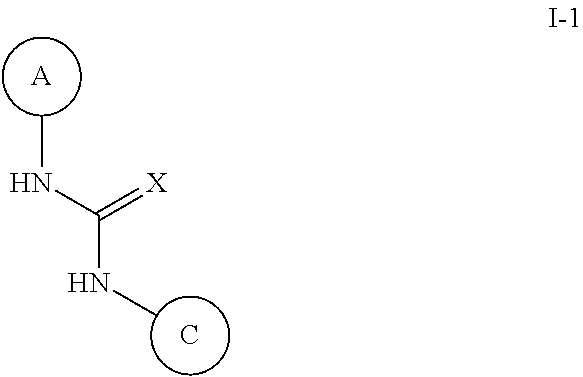
N-(monocyclic aryl),n'-pyrazolyl-urea, thiourea, guanidine and cyanoguanidine compounds as trka kinase inhibitors
Compounds of Formula (I) or stereoisomers, tautomers, or pharmaceutically acceptable salts, solvates or prodrugs thereof, wherein Ring A, Ring C and X are as defined herein, are inhibitors of TrkA kinase and are useful in the treatment of diseases which can be treated with a TrkA kinase inhibitor such as pain, cancer, inflammation / inflammatory diseases, neurodegenerative diseases, certain infectious diseases, Sjogren's syndrome, endometriosis, diabetic peripheral neuropathy, prostatitis or pelvic pain syndrome.
Owner:ARRAY BIOPHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com