Process for preparing heat-labile enterotoxin of E, coli
A heat-labile, Escherichia coli technology, applied in the biological field, can solve the problems of limiting the large-scale production and clinical application of LT and its mutants, low LTB yield, low yield, etc., to avoid endotoxin contamination and low production costs. , the effect of improving the recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] - Intracellular expression of LT in Pichia pastoris cells
[0040] (1) Construction of recombinant expression vectors LTA / pA0815 and LTB / pA0815
[0041] The LTA and B subunit genes use their upstream and downstream specific primers to amplify the genes without their signal peptide sequences respectively, and introduce EcoR I enzyme cutting points at both ends of the primers. The PCR product sizes are LTA (727bp), LTB (320bp ) was purified and digested with EcoR I at 37°C for 2 hours, recovered and purified; the yeast expression vector pA0815 was also digested with EcoR I, then treated with alkaline phosphatase at 37°C for 1 hour, extracted with phenol / chloroform, and precipitated with ethanol. TE suspension carrier DNA fragments, and EcoR I-digested LTA and LTB were ligated with T4 DNA ligase at 16°C overnight, and the ligated products were transformed with CaCl by heat shock method 2 Prepared E.coli DH5α, coated with Amp + -LB plate, cultured at 37°C for 16 hours, pi...
Embodiment 2
[0050] ——Construction and identification of LT secreted and expressed by Pichia pastoris
[0051] (1) Construction of recombinant expression vectors αLTA / pA0815 and αLTB / pA0815
[0052] The LTA and B subunit genes without signal peptide sequences were amplified by PCR respectively, and Xho I and EcoR I restriction sites were introduced at the upper and lower ends of the primers, and the PCR products LTA (727bp) and LTB (328bp) were purified and used Xho I and EcoR I were digested at 37°C for 2 hours, recovered and purified; the recombinant vector pALPHA containing the yeast secretion signal peptide α-factor was also double digested with Xho I and EcoR I, the large fragment was recovered from the gel, and the double digested and purified LTA and LTB were ligated overnight at 16°C with T4 DNA ligase, and the ligated products were transformed by heat shock method with CaCl 2Prepared E.coli DH5α, coated with Amp + -LB plate, cultured at 37°C for 16 hours, picked several single c...
Embodiment 3
[0060] ——Intracellular expression and secretory expression of LTB subunits in Pichia pastoris cells
[0061] (1) Construction, induced expression and identification of LTB expressed in Pichia pastoris cells
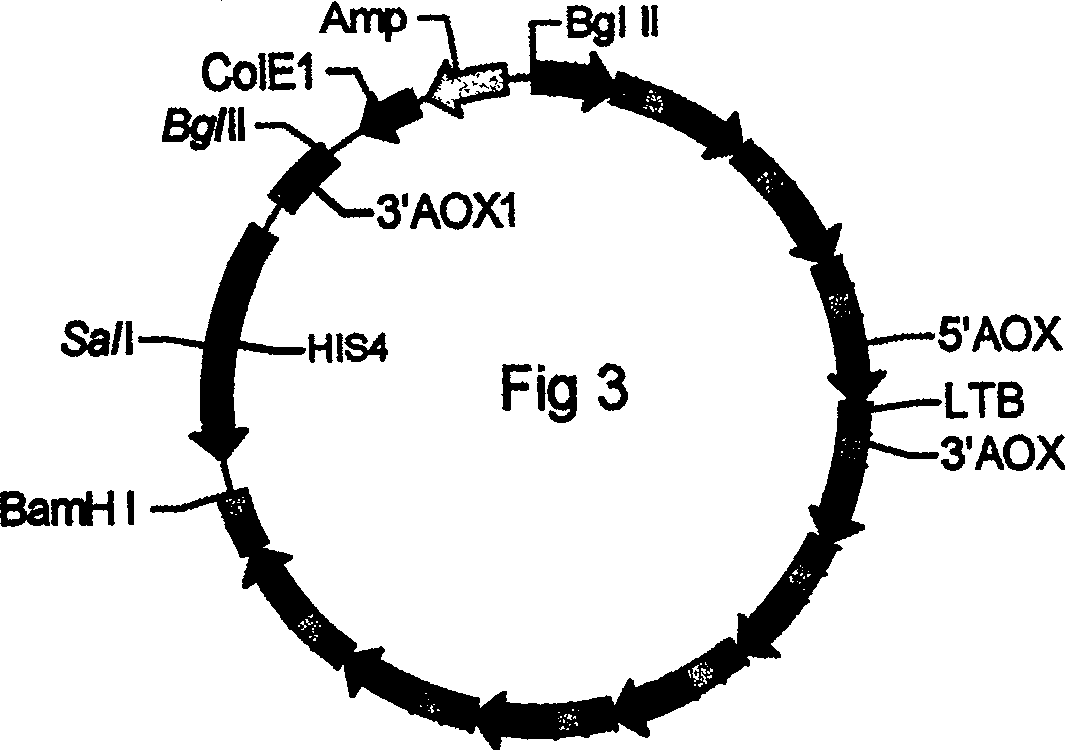
[0062] Construct successful 5 copy expression cassettes (AOX1-LTB) in embodiment 1 5 / pA0815 recombinant plasmid, continue to construct 10 copies of expression cassette (AOX1-LTB) according to the method in embodiment 1 10 / pA0815 recombinant plasmid, electrotransformation of Pichia pastoris GS115, identification of Pichia pastoris transformants (the fragment amplified with primers 5AOX and 3AOX is 514bp), induction of expression with methanol, purification by affinity chromatography, and SDS-PAGE and Western blot Identification. The size on 10% SDS-PAGE gel is 11KD, and the intracellular expression yield of LTB is 116.8 mg / L.
[0063] (2) Construction, induced expression and identification of LTB secreted and expressed in Pichia pastoris cells
[0064] The successful...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com