Method for preparing faropenem
A technology for faropenem sodium and sodium isooctanoate is applied in the field of preparing antibiotic drug faropenem sodium, and can solve the problems of long operation steps, high price, unsuitability for industrialized production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
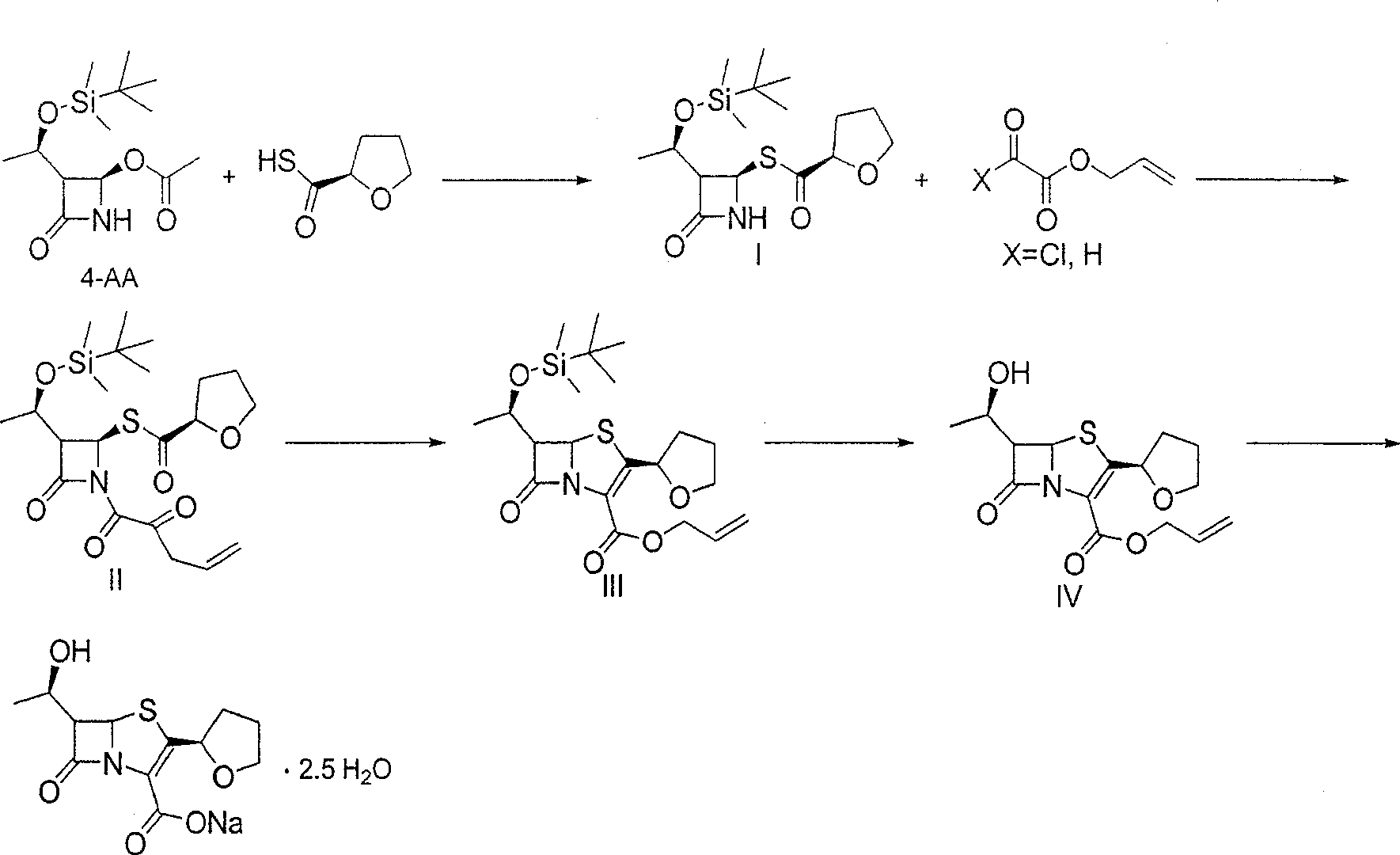
[0020] (3R,4R)-3-[(R)-1-tert-butyldimethylsiloxyethyl]-4-[(R)-tetrahydrofuran-2-formylthio]azetidin-2- Preparation of ketone (I)
[0021] Method A:
[0022] Dissolve 184g (1.394mol) of R-(+)-thiotetrahydrofuran-2-carboxylic acid in 200ml of dry 1,4-dioxane, and dissolve 193ml (1.394mol) of triethylamine in 1,4-dioxane Oxycycline 50ml was added dropwise to the above solution under cooling, the pH value was 9 after dropping, and the stirring was continued for 0.5 hour, and set aside.
[0023] (3R, 4R)-3-[(R)-1-tert-butyldimethylsiloxyethyl]-4-[(R)-acetoxy]azetidin-2-one (4- AA) 200g (0.697mol) was dissolved in 800ml of dry 1,4-dioxane, 142g (1.045mol) of dry zinc chloride was added at 20°C, and after stirring for 15 minutes, the R-( +)-Triethylamine salt of thiotetrahydrofuran-2-carboxylic acid, the rate of addition is controlled so that the temperature of the internal bath is not higher than 35°C. After dropping, the reaction was continued at 35°C for 8 hours. Stop the rea...
Embodiment 2
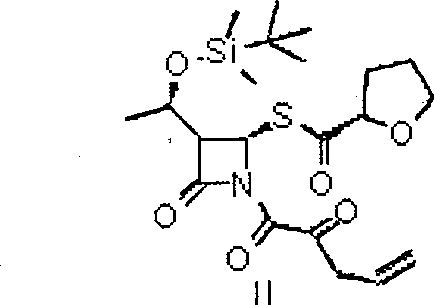
[0027] (3R,4R)-1-allyloxyoxalyl chloride-3-[(R)-1-tert-butyldimethylsiloxyethyl]-4-[(R)-tetrahydrofuran-2-formyl Preparation of Sulfuryl]azetidin-2-one (II)
[0028] Method A:
[0029] Dissolve 220 g (0.613 mol) of intermediate I in 1000 ml of dry dichloromethane, cool to -10°C, add 145.6 g (0.980 mol) of monoallyloxyoxalyl chloride dropwise, and after the drop is complete, dissolve 145 ml of triethylamine ( 1.04mol) was dissolved in 200ml of dichloromethane and added dropwise to the above reaction solution, the rate of addition was controlled so that the temperature of the internal bath was not higher than -10°C. After dripping, keep the reaction for 1.5 hours, stop the reaction, wash the organic phase with water 200ml×1, wash the organic phase with 5% sodium bicarbonate solution to be neutral, wash with 10% sodium chloride solution 200ml×1, dry over anhydrous sodium sulfate, concentrate, 260 g of light yellow oily substance II was obtained.
[0030] Method B:
[0031] Di...
Embodiment 3
[0033] (5R,6S)-6-[(R)-1-tert-butyldimethylsiloxyethyl]-2-[(R)-tetrahydrofuran-2-formylthio]penem-3-carboxylate Preparation of Acryl Acrylate (III)
[0034]Dissolve 260g (0.552mol) of intermediate II in 400ml of xylene, add 229g (1.380mol) of triethyl phosphite, add 1g of hydroquinone, reflux for 1.5 hours, wash the reaction solution with water 100ml×3, anhydrous sodium sulfate Drying and concentration gave a residue of 184 g. After column chromatography (silica gel column, petroleum ether / ethyl acetate 15:1), 150 g of a light yellow solid was obtained, with a yield of 49% (based on 4-AA), mp63-65 ℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com