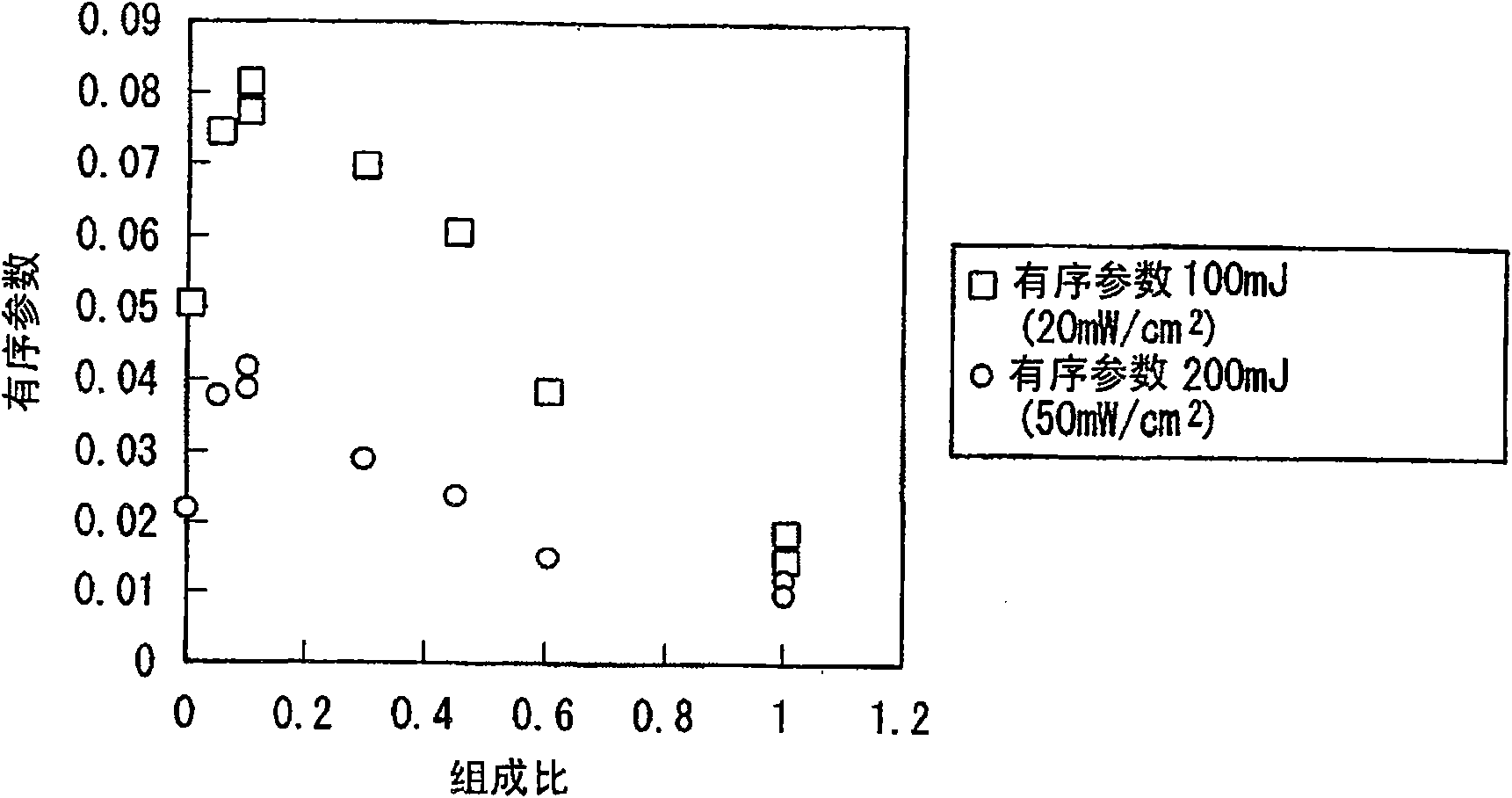
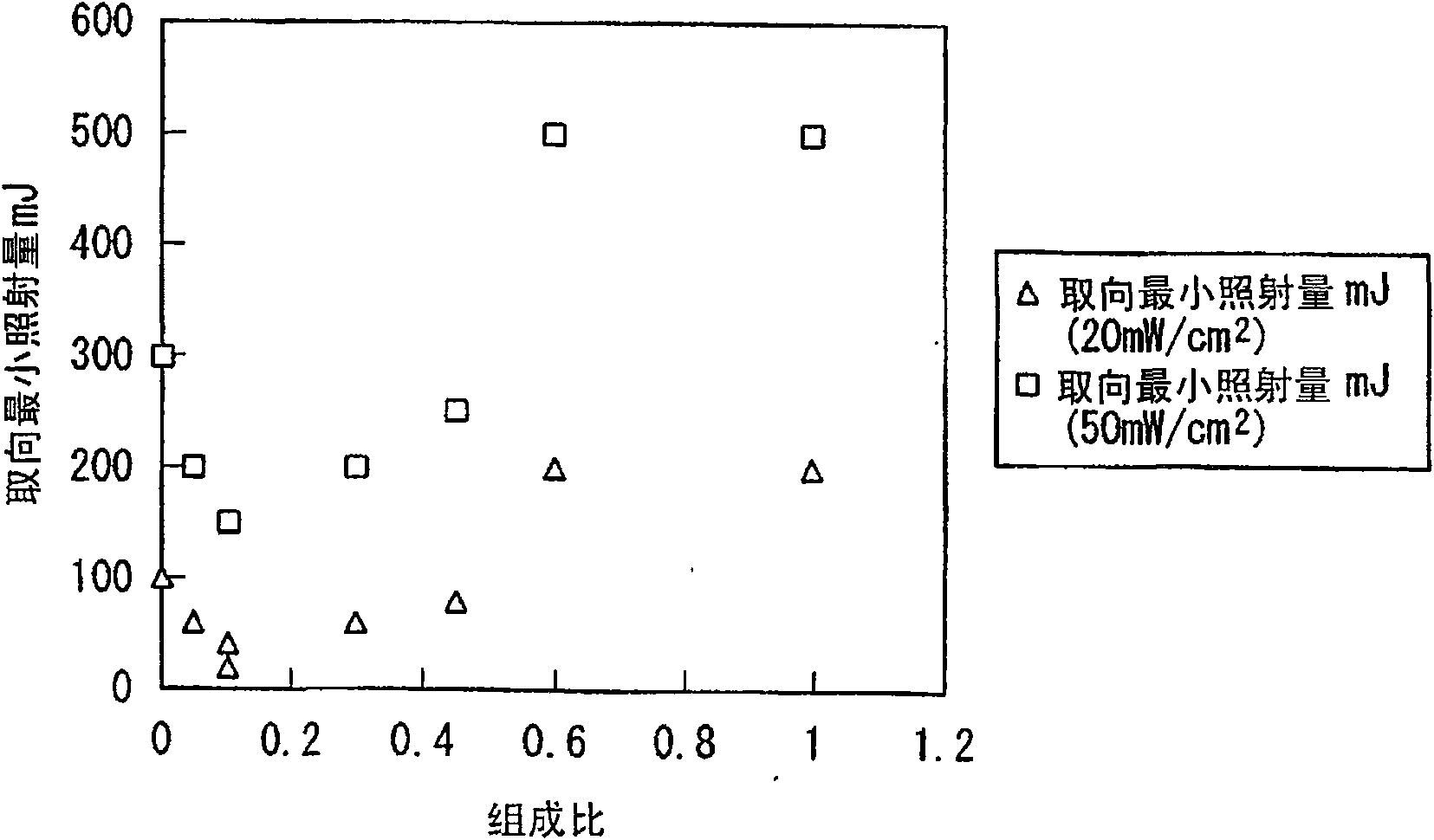
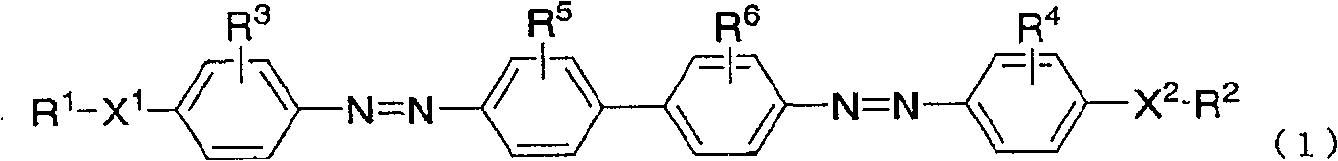
Azo compound, composition for optical alignment film using same, and method for producing optical alignment film
An azo compound and photo-alignment film technology, which is applied in acylation preparation, disazo dyes, optics, etc., can solve the problems of screen flicker and low voltage retention rate of photo-alignment film, and achieve high affinity and voltage retention. Good rate and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] (Example 1) Synthesis of compound No.A-1
[0130] Dissolve 5.00 g (14.5 mmol) of 2,2'-benzidine disulfonic acid in 70 ml of 3.3% (w / v) sodium hydroxide aqueous solution, and stir at 0 to 5°C. Maintain the temperature, and add 2.10 g (30.5 mmol) of sodium nitrite dissolved in 60 ml of water, and then slowly drop in 22 ml of 8N aqueous hydrochloric acid. After the dripping, the temperature of the reaction solution was maintained and stirring was continued for 3 hours to prepare a diazonium salt mixture. Then, dissolve 4.0g (29.0mmol) of 2-ethoxyphenol in 140ml of 3.5% (w / v) sodium hydroxide aqueous solution, cool to 0~5℃, stir and slowly drop in the diazonium obtained by the above method Salt mixture. After the dropping, the temperature of the reaction liquid was maintained and stirring was continued for 2.5 hours. 70 g of sodium chloride was added to the reaction solution, stirred for a while at room temperature, and the formed precipitate was filtered to obtain a crude ...
Embodiment 2
[0131] (Example 2) Synthesis of Compound No. A-7
[0132] Dissolve 1.50 g (4.35 mmol) of 2,2'-benzidine disulfonic acid in 21 ml of 3.3% (w / v) sodium hydroxide aqueous solution, and stir at 0 to 5°C. The temperature was maintained, and 0.633 g (9.17 mmol) of sodium nitrite dissolved in 15 ml of water was added, and then 6.54 ml of 8N aqueous hydrochloric acid was slowly dropped. After the dropping, the temperature of the reaction solution was maintained and stirring was continued for 3 hours to prepare the diazonium salt. Then, 1.34g (8.70mmol) of 2-methoxyphenol dissolved in 2ml of tetrahydrofuran was dissolved in 85ml of 5% (w / v) sodium carbonate aqueous solution, then cooled to 0~5℃, stirred and slowly dripped The diazonium salt mixture obtained by the above method. After the dropping, the temperature of the reaction liquid was maintained and stirring was continued for 2.5 hours. 28 g of sodium chloride was added to the reaction solution, stirred for a while at room tempera...
Embodiment 3
[0133] (Example 3) Synthesis of Compound No. A-10
[0134] Dissolve 2.75g (3.83mmol) of compound No.A-7 obtained in Example 2 and 2.24g (7.67mmol) 4-(6-acryloyloxyhexyloxy) in 30ml of N,N-dimethylformamide Benzoic acid, 1.54g (8.02mmol) 1-ethyl-3-(3'-dimethylaminopropyl) carbodiimide hydrochloride, cool and stir in an ice bath, slowly add 330mg (2.70mmol) ) A solution of 4-(N,N-dimethylamino)pyridine dissolved in 5ml of N,N-dimethylformamide. The ice bath was removed, and after stirring for 3 hours at room temperature, the reaction solution was poured into water-0.5N hydrochloric acid water and extracted with dichloromethane. The organic layer was washed with half-saturated brine, and dried over anhydrous magnesium sulfate. The organic layer was filtered, and the remaining part obtained by concentrating the filtrate under reduced pressure was purified by silica gel column chromatography (eluent dichloromethane / methanol / acetic acid = 40 / 1 / 1 to 20 / 1 / 1) and collected After the fr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com