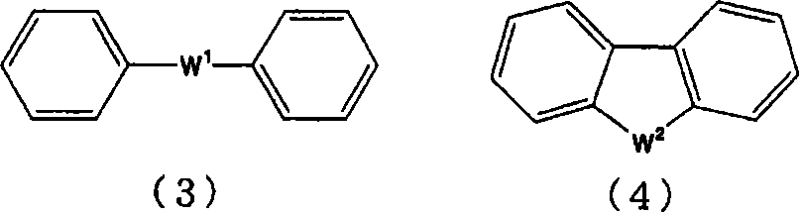
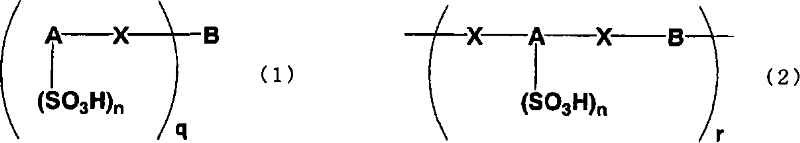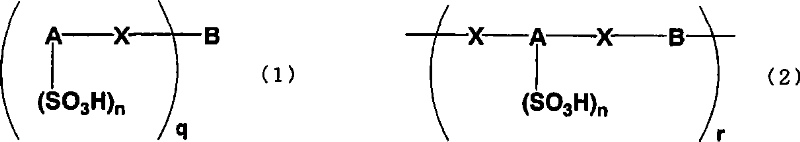Arylsulfonic acid compound and use thereof as electron-acceptor material
An aryl sulfonic acid and compound technology, applied in the field of aryl sulfonic acid compounds, can solve the problems of reduced characteristics, limited selection range, uneven surface unevenness, and achieves the effects of reducing driving voltage, improving current efficiency, and uniform light-emitting surface
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] Naphthalene disulfonic acid compound oligomer 1 (hereinafter abbreviated as NSO-1) was synthesized according to the following reaction formula (14).
[0150]
[0151] That is, in a nitrogen atmosphere, 449 mg of perfluorobiphenyl, 60% hydrogenated 161 mg of sodium and 50 ml of anhydrous N,N-dimethylimidazolidinone were replaced with nitrogen in the reaction system, and stirred at 80° C. for 43 hours.
[0152] After cooling to room temperature, add water to stop the reaction, reduce pressure, concentrate and evaporate to dryness. 5 ml of methanol was added to the residue, and the resulting suspension was added to 100 ml of diethyl ether while stirring. After stirring at room temperature for 1 hour, the precipitated solid was collected by filtration, 50 ml of methanol was added to the filtrate, and the mixture was suspended by ultrasonication. The insoluble solid was removed by filtration, and the filtrate was concentrated under reduced pressure and evaporated to dry...
Embodiment 2
[0157] Naphthalene disulfonic acid compound oligomer 2 (hereinafter abbreviated as NSO-2) was synthesized according to the following reaction formula (15).
[0158]
[0159] That is, in a nitrogen atmosphere, 450 mg of perfluorobiphenyl and 166 mg of 60% sodium hydride were sequentially added to 934 mg of fully dried sodium 1-naphthol-3,6-disulfonate (manufactured by Tokyo Chemical Industry Co., Ltd.) and 50 ml of anhydrous N,N-dimethylimidazolidinone, the inside of the reaction system was replaced with nitrogen, and stirred at 80°C for 43 hours.
[0160]After cooling to room temperature, water was added to stop the reaction, and concentrated under reduced pressure and evaporated to dryness. 5 ml of methanol was added to the residue, and the resulting suspension was added to 100 ml of diethyl ether while stirring. After stirring at room temperature for 1 hour, the precipitated solid was collected by filtration, and 25 ml of methanol was added to the filtrate, followed by u...
Synthetic example 1
[0164] [Synthesis Example 1] Synthesis of Phenyltetraphenylamine
[0165]
[0166] Based on the method described in Bulletin of Chemical Society of Japan, 1994, Vol. 67, p. 1749-1752, phenyltetraphenylamine (PTA) was produced in the following manner.
[0167] That is, 12.977 g of p-phenylenediamine was dissolved in 2 L of toluene, 245.05 g of tetra-n-butoxytitanium as a dehydration condensation agent was added thereto, and dissolved at 70° C. for 30 minutes. Thereafter, 53.346 g of p-hydroxydiphenylamine was added, and reacted at a reaction temperature of 100° C. for 24 hours under a nitrogen atmosphere. After the reaction, the reaction liquid was filtered, and the filtrate was washed with toluene and diethyl ether in sequence, and dried to obtain silver crystals. 25 parts by mass of dioxane and 0.2 equivalent of hydrazine monohydrate were added to the obtained crystals, and the inside of the reaction system was replaced with nitrogen, followed by heating to reflux to diss...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com