Depolymerization glycosaminoglycan extracted from sea cucumber composition and its preparation method and application
A technology of glycosaminoglycans and compositions, which is applied in the field of prevention and treatment of cardiovascular and cerebrovascular diseases, and can solve the problem that the safety, effectiveness and quality of low-depolymerized sea cucumber glycosaminoglycans cannot meet the pharmaceutical standards , Difficult to remove fucose from sea cucumbers, etc., to achieve anticoagulant mechanism and excellent safety, prevention and treatment of coagulation and thrombosis diseases, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
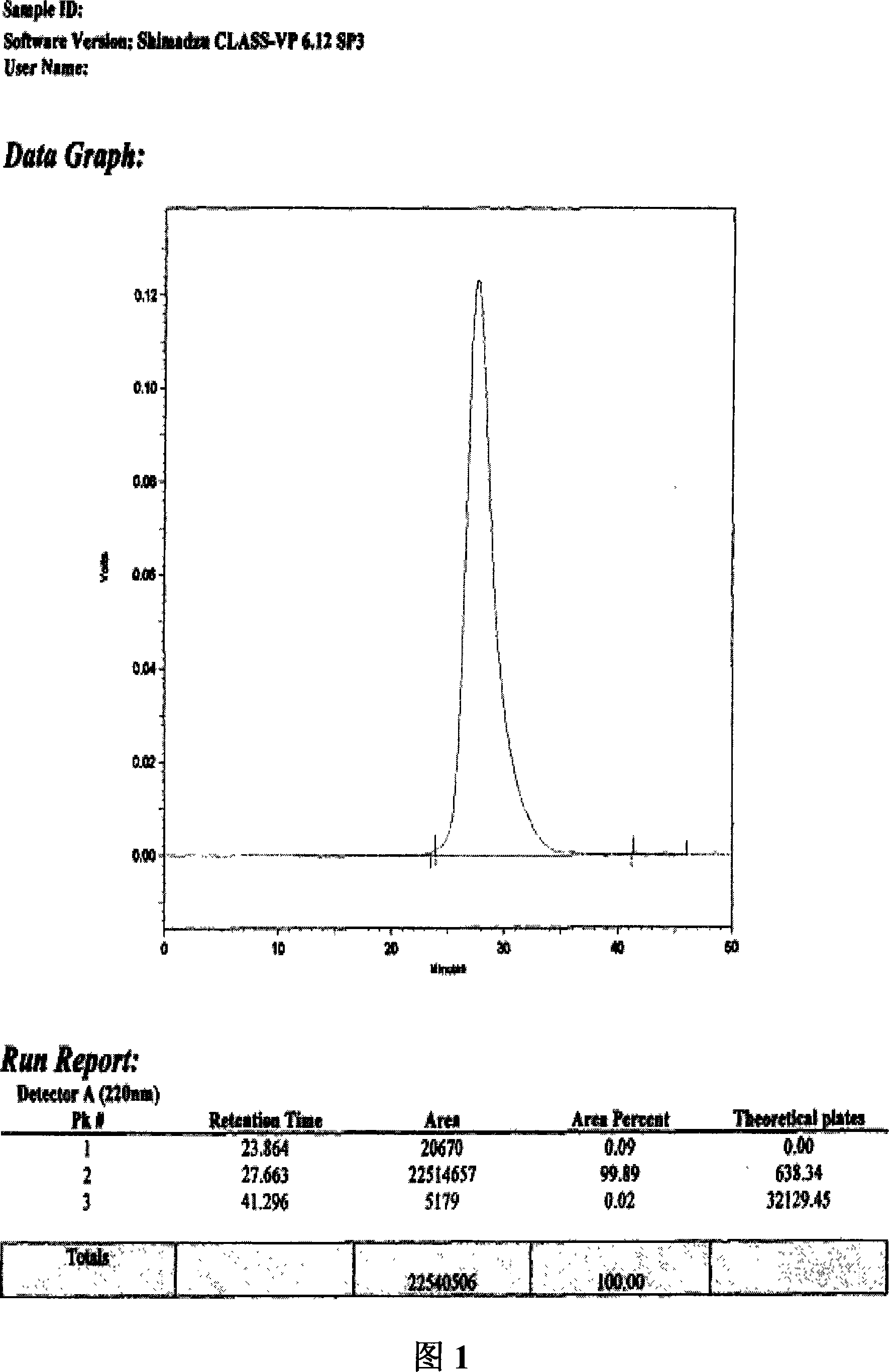
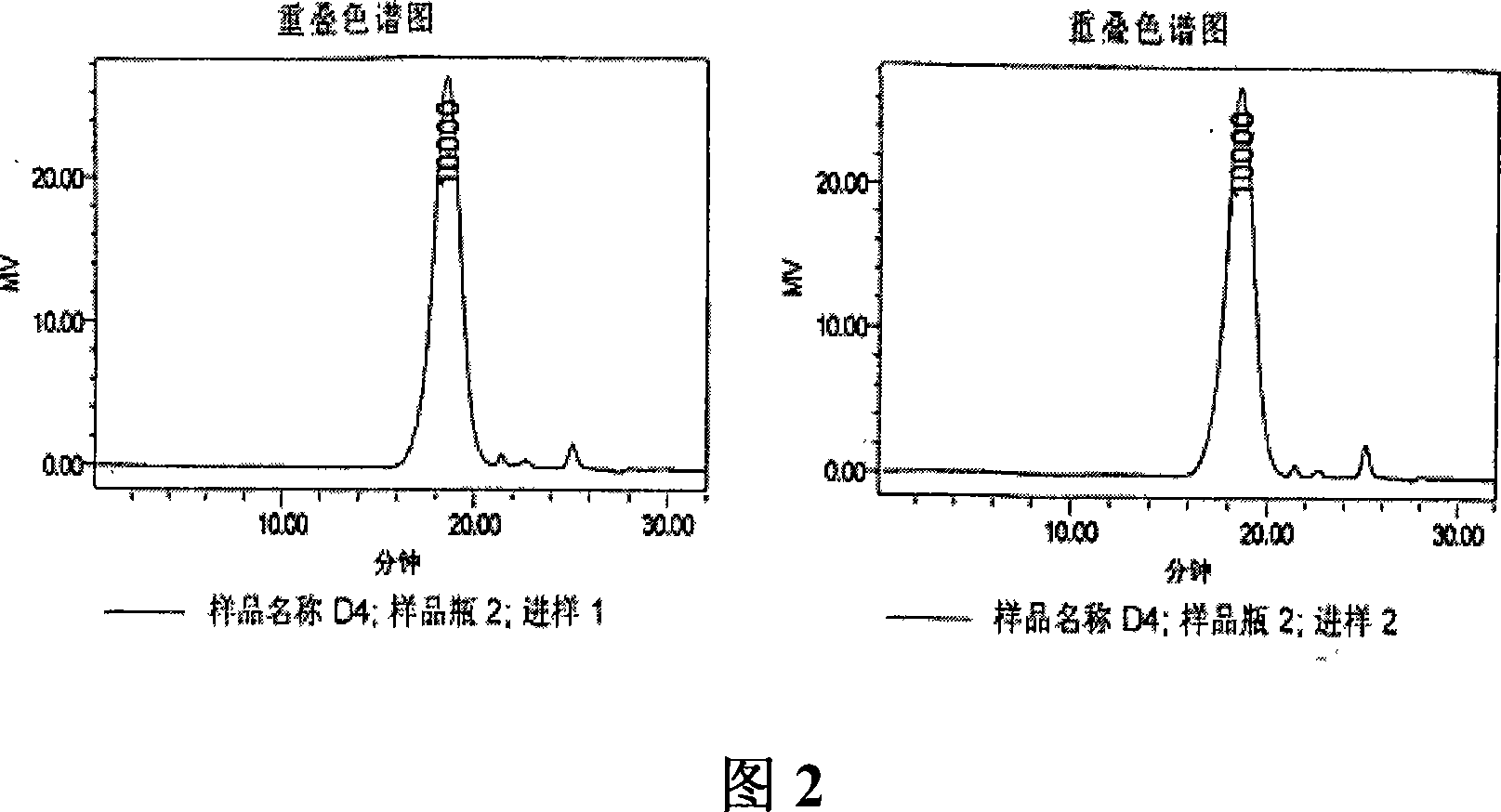
[0041] In a preferred embodiment of the preparation of the depolymerized sea cucumber glycosaminoglycan composition of the present invention, in order to obtain a high-purity depolymerized black sea cucumber glycosaminoglycan composition suitable for intravenous injection, the dried black sea cucumber body wall can be twisted successively crushed, centrifuged after enzymatic hydrolysis, the supernatant was precipitated with ethanol, and dried to obtain the precipitate ①; after the precipitate ① was dissolved in water, calcium chloride was added, centrifuged, the supernatant was precipitated with ethanol, and dried to obtain the precipitate ② Dissolve the precipitate ② in water, add potassium acetate, take the supernatant, add ethanol to precipitate, and dry to obtain the crude sea cucumber polysaccharide; pass the crude sea cucumber polysaccharide through a DEAE-cellulose column, use ethanol to precipitate, add hydrogen peroxide to decolorize the heat source , the sea cucumber ...
Embodiment 1
[0047] 1.1 Extraction of sea cucumber polysaccharides
[0048] Take 1000g of dried black sea cucumber (Holothuria atra) body wall, add water to soak, wash and drain, mince, add water to 8kg, adjust the pH value to 8.0 with sodium hydroxide, add alkaline protease (Wuxi Enzyme Preparation Factory) 20ml , at 60° C. for enzymolysis, after the enzymolysis, 2 g of trypsin (Sichuan Deyang Enzyme Preparation Factory) was added for a second enzymolysis, the enzymolysis product was centrifuged at room temperature, and the supernatant was taken. The pH value was adjusted to 2.5 with hydrochloric acid, and the acidic protein precipitate was removed by centrifugation. Add 0.8 times the volume of ethanol, centrifuge at 4°C, and obtain the supernatant to obtain a precipitate. Add 10 times the weight of the precipitate to dissolve it in water, heat to 90°C, add calcium chloride to make the concentration of calcium chloride 2%, keep it warm for 20 minutes, cool, centrifuge, collect the supern...
Embodiment 2
[0069] Example 2 Pharmacodynamic test of depolymerized sea cucumber glycosaminoglycan
[0070] 1. Experimental materials
[0071] 1.1 Animals: SD strain white rats, ♂ sex, weight 250-320g, quality certificate: Shanyidongzhengzi No. 08-005; New Zealand strain white rabbits, ♀♂ half and half, weight 1.8-2.2kg, quality certificate: Shaanxi Medical Dongzheng No. 08-18.
[0072] 1.2 Drug: depolymerized sea cucumber glycosaminoglycan for injection
[0073] Specifications: 10mg / bottle (based on the active ingredient - depolymerized sea cucumber glycosaminoglycan)
[0074] Positive control drug: Xuesaitong injection (specification: 2ml / 100mg / bottle)
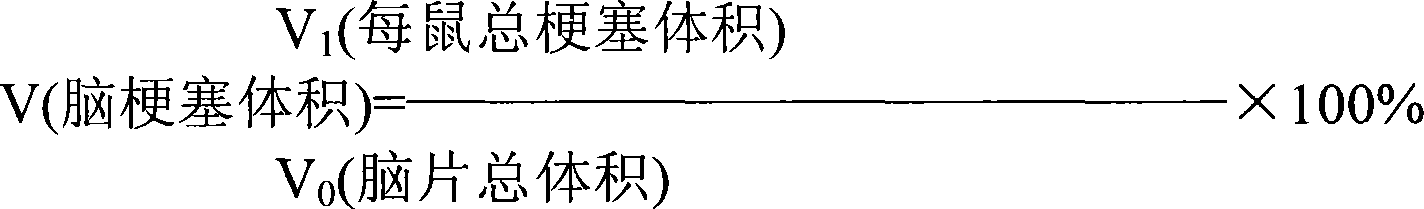
[0075] (1) Effects of Depolymerized Sea Cucumber Glycosaminoglycans for Injection on Rats with Focal Cerebral Ischemia
[0076] (1) Experimental method
[0077] Take the SD strain, half male and half female, with enough rats weighing 280-320g, start weighing after 7 days of adaptation to the laboratory, number them, and randomly div...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com