Engineering bacterium producing 5-glycyl ethylformic acid and construction method thereof
A technology of aminolevulinic acid and its construction method, which is applied in the field of engineering bacteria for producing 5-aminolevulinic acid and its construction, and can solve the problems of high cost, long cycle, low enzyme activity and low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
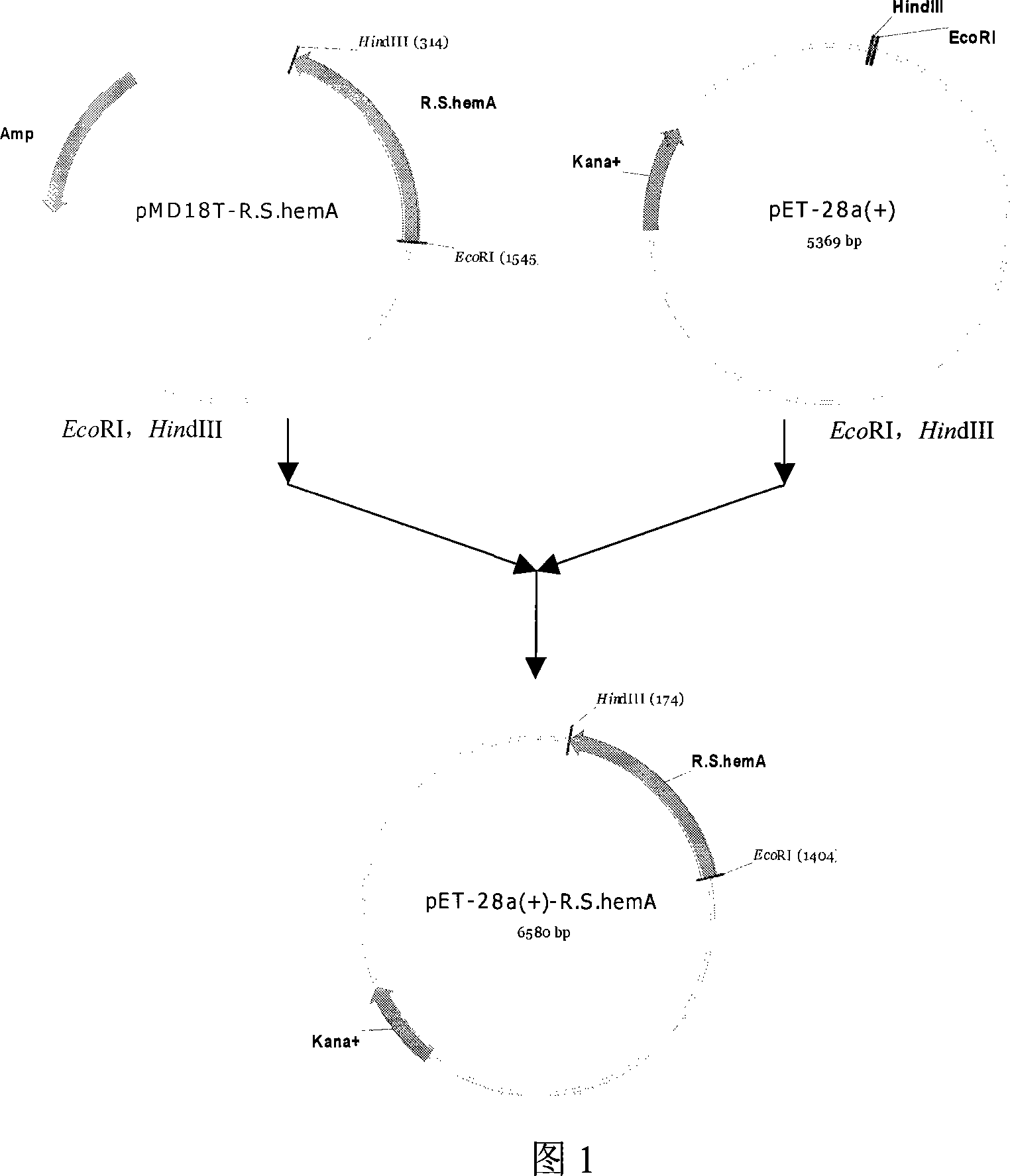
[0020] The construction method of the engineering bacteria producing 5-aminolevulinic acid of the present invention comprises the following steps:
[0021] 1. Extraction of Rhodobacter sphaeroides genomic DNA
[0022] 1) Put the overnight cultured bacterial solution in 1.5ml centrifuge, centrifuge at 13,000×g for 1min and remove the supernatant;
[0023] 2) After dissolving with 500 μl TE buffer solution, add 30 μl 10% (w / v) sodium dodecyl sulfate (SDS) solution, and at the same time add 3 μl 20 mg / ml proteinase K, mix well, and incubate at 37°C for 1 hour;
[0024] 3) Add 1 / 5 volume of 5mol / L NaCl solution, plus about 1 / 5 volume of CTAB / NaCl solution (4.1g NaCl dissolved in 80ml H 2 O, slowly add 10g CTAB, add water to 100ml), keep warm at 65°C for 30min;
[0025] 4) Add an equal volume of a mixture of phenol, chloroform and isoamyl alcohol (the volume ratio of phenol: chloroform: isoamyl alcohol is 25:24:1), and centrifuge at 13,000×g for 5 min;
[0026] 5) Take the super...
Embodiment 2
[0045] Expression of 5-aminolevulinic acid synthase under the action of inducer isopropyl-β-D-thiogalactoside (IPTG)
[0046] 1) Cultivate the engineering bacterium Rosetta(DE3)-pET28a-R.S.hemA of the present invention in a 250ml shake flask containing 50ml LB medium, first culture at 37°C and 200rpm for 2.0h; then cool down to 28°C and use 2.5μl Induced by 1mol / L IPTG, after continuing to cultivate for 6 hours, take 30ml of bacterial liquid and centrifuge at 10,000×rpm for 10min to remove the supernatant;
[0047] 2) Wash once with 9ml 50mmol / L Tris Cl (pH 7.5), resuspend with the same amount of buffer, and disrupt the cells by ultrasonication. The conditions are 300w for 5s, 7s for 30 repetitions, and centrifugation at 10,000×rpm 10min;
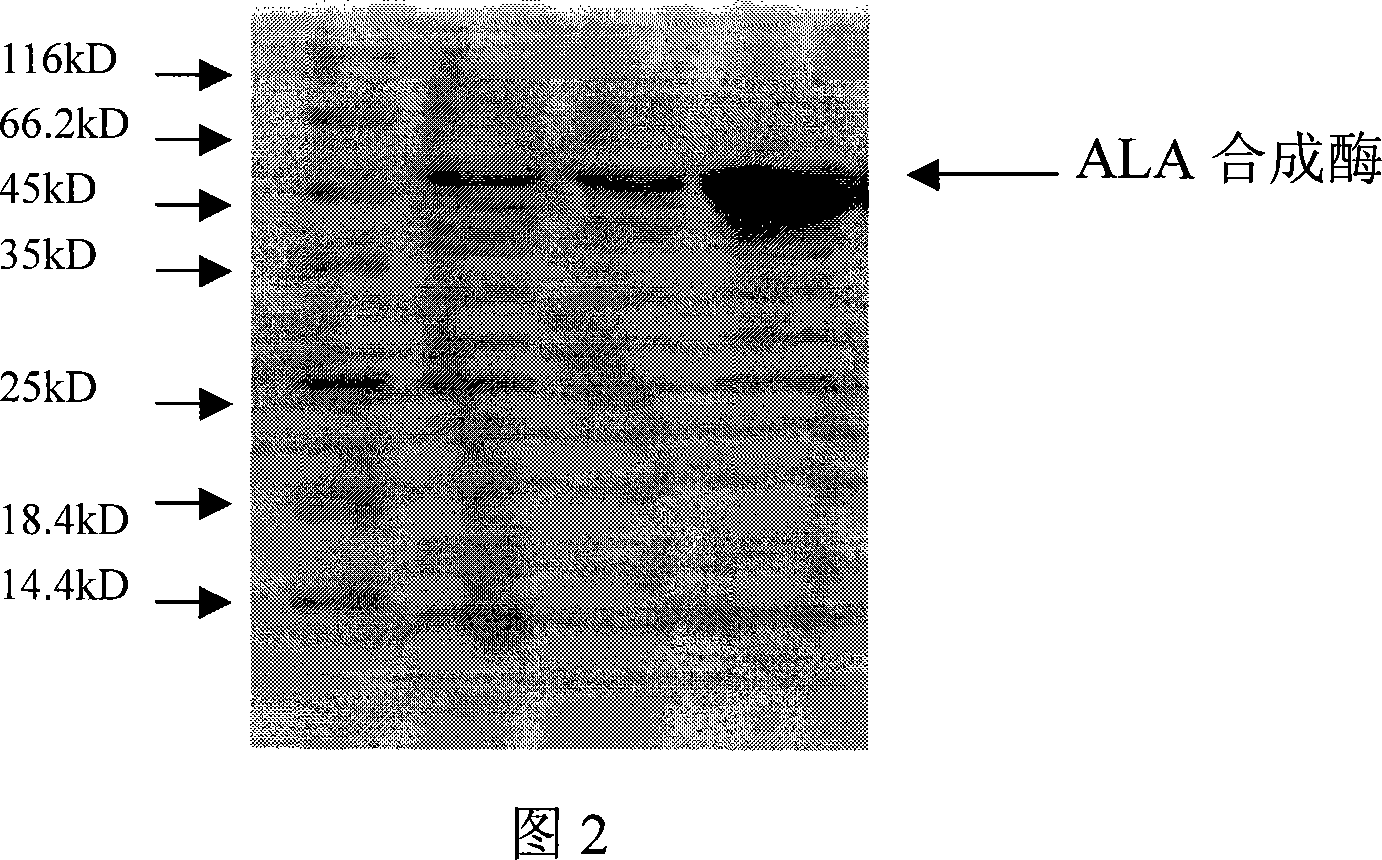
[0048] 3) Take the supernatant and carry out 10% SDS-polyacrylamide gel electrophoresis;
[0049] 4) According to the electrophoresis results, there is a soluble protein expression accounting for 25% of the total protein amount at about 5...
Embodiment 3
[0051] Determination of specific activity of 5-aminolevulinic acid synthase
[0052] 1) 500μl containing 50mmol / L Tris-HCl (pH7.5), 20mmol / L MgCl 2 , 0.1mol / L glycine, 0.27mmol / L pyridoxal phosphate, 0.2mmol / L succinyl-CoA and the mixed solution of the cell supernatant after cell destruction, react at 37°C for 10min;
[0053] 2) Add 150 μl of 10% trichloroacetic acid to the above reaction solution, mix in a 1.5 mL centrifuge tube, and centrifuge at 13,000×g for 5 min;
[0054] 3) Mix 300 μl supernatant with 400 μl 1mol / L acetate buffer (pH 4.6) and 35 μl acetylacetone, and bathe in boiling water for 15 minutes;
[0055] 4) After cooling to room temperature for 15 min, add 700 μl of freshly prepared Ehrlich reagent (add 30 ml of glacial acetic acid, 1 g of p-dimethylaminobenzaldehyde, 5 ml of 70% perchloric acid, 5 ml of water in turn, dissolve and fix with glacial acetic acid to 50ml), after reacting for 30min, measure its absorbance at 554nm, the concentration of ALA (mg / L)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com