Tissue factor production inhibitor
A tissue factor and drug technology, applied in the direction of pill delivery, drug combination, medical preparations containing active ingredients, etc., can solve the problem of unknown role of LXR tissue factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1009] Compounds A to J in Table 7 of Example 1 and Table 8 of Example 2 represent the following compounds.
[1010] Compound A: N-(2,2,2-trifluoroethyl)-N-{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl }Benzenesulfonamide (compound 12 described on page 55 of WO2000 / 054759)
[1011] [chem 25]
[1012]
[1013] Compound B: 3-chloro-4-(3-(2-propyl-3-trifluoromethyl-6-benzene-[4,5]-isoxazolyloxy)propylthio)phenylacetic acid (WO1997 / 028137 The compound described in Example 20 on page 70; the effect on LXR is described in Endocrinology, 143, pp.2548-2558, 2002)
[1014] [chem 26]
[1015]
[1016] Compound C: 2-(3-{3-[[2-chloro-3-(trifluoromethyl)benzyl](2,2-diphenylethyl)amino]propoxy}-phenyl)acetic acid (The compound described in Example 16 on page 46 of WO2002 / 24632)
[1017] [chem 27]
[1018]
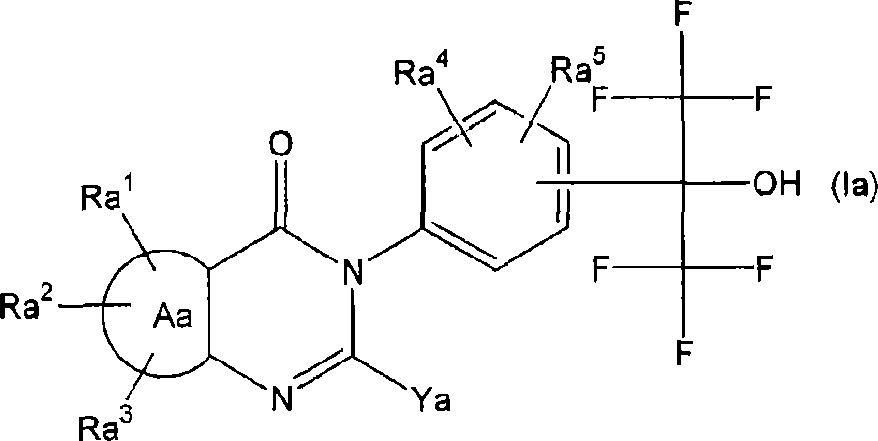
[1019] Compound D: 2-benzyl-6,7-dimethoxy-3-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl] -4(3H)-quinazolinone (the compound described in...
Embodiment 2
[1067] (Example 2) LPS-administered mouse tissue factor mRNA analysis
[1068] The test compound was dissolved in a solution obtained by mixing propylene glycol (Wako Pure Chemical Industries, Ltd.) and Tween 80 (Kao) at a ratio of 4:1 (hereinafter referred to as "PG / Tween"). Male C57BL / 6T mice (Charles River ) for mandatory oral administration for 7 days, once a day in the evening, with a dose of 10 mg / kg. LPS 4 mg / kg was intraperitoneally administered at 9:00 am on the second day after the seventh administration, and 6 hours later, the abdomen was opened under ether anesthesia, and the kidney was removed. Kidney RNA was extracted with Trizolregent (Invitrogen). After performing reverse transcription reaction on the obtained RNA using First-Strand cDNA Synthesis Kit, the expression levels of tissue factor mRNA and cyclophilin mRNA were measured by quantitative RT-PCR in the same manner as in Test Example 1 above. Table 8 shows the tissue factor mRNA expression level when on...
reference example 1
[1098] (Reference Example 1) 6-Chloro-7-methoxy-3-{2-methyl-5-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl] Phenyl}-2-(3-ethynylmethyl)-4(3H)-quinazolinone (quinazolinone)
[1099] Operate in the same manner as the method described in the document (Example 1 on page 271 of WO2003 / 106435), and the 5-chloro-4-methoxy-ortho Aminobenzoic acid (201mg, 1.0mmol), phenylacetic acid (142mg, 1.0mmol), triphenyl phosphite (0.29ml, 1.1mmol) and the embodiment 147 (1 )] 2-(3-amino-4-methylphenyl)-1,1,1,3,3,3-hexafluoro-2-propanol (273mg, 1.0mmol) synthesized by the method described, prepared The title compound (344 mg, yield 61%) was obtained as a colorless solid.
[1100] 1 H-NMR (500MHz, DMSO-d 6 ): δ8.89 (1H, br), 8.06 (1H, s), 7.78 (1H, s), 7.70 (1H, d, J = 8.0Hz), 7.42 (1H, d, J = 8.0Hz), 7.34 -7.41(2H, m), 6.70(1H, s), 6.59(1H, d, J=5.0Hz), 4.05(3H, s), 3.81(1H, d, J=15.0Hz), 3.76(1H, d,J=15.0Hz), 1.63(3H,s).
[1101] ESI(ES+)(m / z): 563([M+H] + ), ESI(ES-)(m / z): 561 ([...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com