Method of preparing 23,24-dihydrocucurbitacin B and use of the same in medicament for treating tumour
A technology of cucurbitacin and dihydrogen, which is applied in the field of medicine, achieves the effects of high-efficiency preparation, inhibition of tumor cell proliferation, and large amount of preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: High-purity preparation of 23,24-dihydrocucurbitacin B
[0028] Firstly, the fresh Trichosanthes root (3950g) was lyophilized and then extracted with petroleum ether (2L) and ethyl acetate (3L). The resulting ethyl acetate extract was removed from the solvent at low temperature to obtain an ethyl acetate extract. Then get 2.5g ethyl acetate extract and carry out preliminary separation with three-axis vertical countercurrent chromatography (developed by Zhejiang University Siyuan Natural Medicine and Biotoxin Research Center, column capacity 600ml), and the two-phase solvent system used is sherwood oil, chloroform, Acetonitrile (volume ratio, 6:1:3), then the fractions were combined. Finally, the cytotoxic components in the collected components are prepared by C8 or C18 reversed-phase high-performance liquid chromatography, and the mobile phase is 10%-100% methanol-water gradient elution, the target substance is collected, and the solvent is volatilized to ob...
Embodiment 2
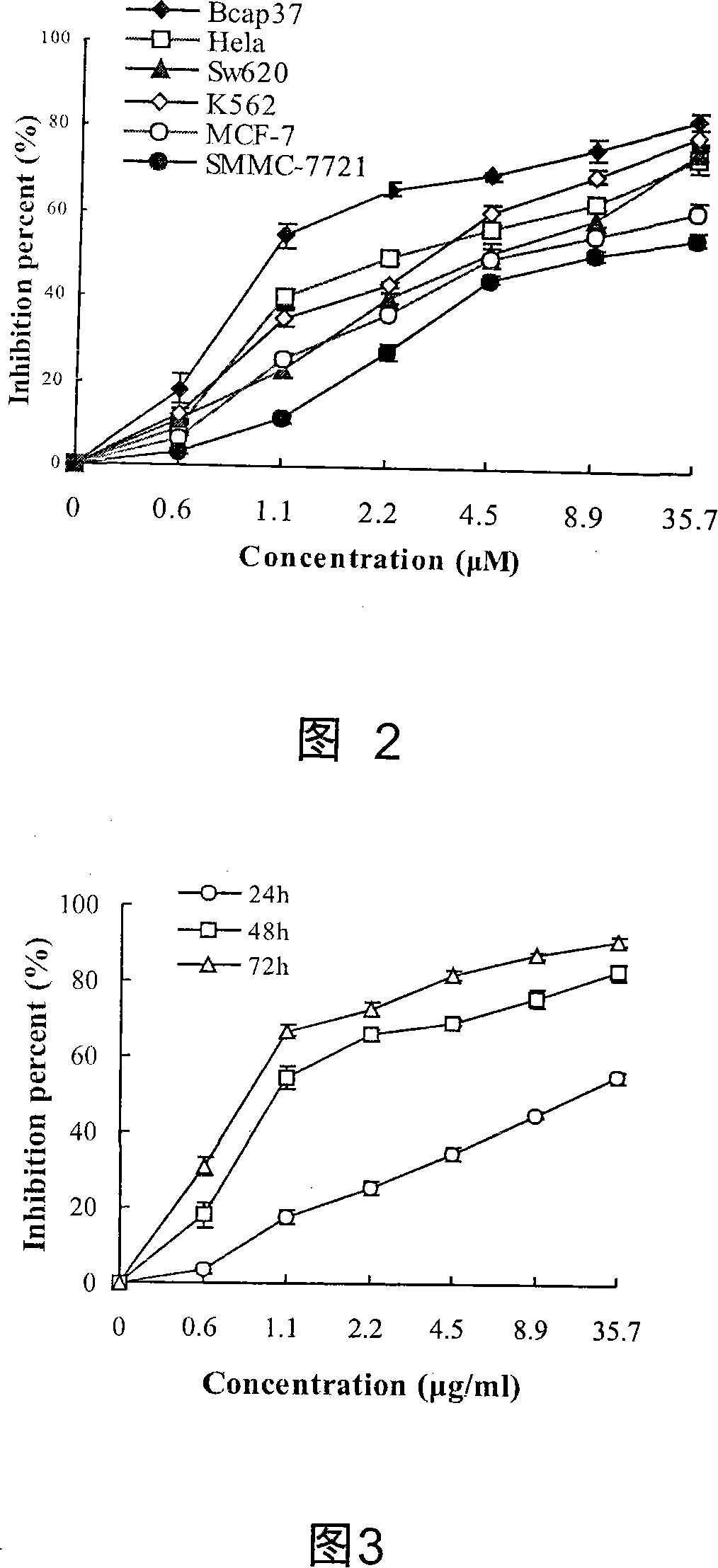
[0029] Example 2: 23,24-Dihydrocucurbitacin B inhibits the proliferation of human tumor cell lines
[0030] experimental method
[0031] (1) Human tumor cell lines and sources: A total of six tumor lines were made, namely breast cancer Bcap37, breast cancer MCF-7, cervical cancer HeLa, liver cancer SMMC-7721, colorectal cancer SW620, and erythroleukemia K562, all from Zhejiang University Cancer Institute.
[0032] (2) 23,24-Dihydrocucurbitacin B was dissolved in DMSO to make 1 mg / ml mother solution, placed in -20°C refrigerator, and diluted with culture solution to final concentrations of 0, 0.6, 1.1, 2.2, 4.5, 8.9, 35.7μM for use, MTT (AMRESCO company product) with phosphate-buffered saline (Phosphate-buffered Saline referred to as PBS) to prepare a 2mg / ml mother solution, placed in a 4 ℃ refrigerator for later use.
[0033](3) Cell culture: all cells are cultivated in RPMI-1640 complete medium, (SIGMA), add 10% newborn bovine serum (Hangzhou Sijiqing Bioengineering Materia...
Embodiment 3
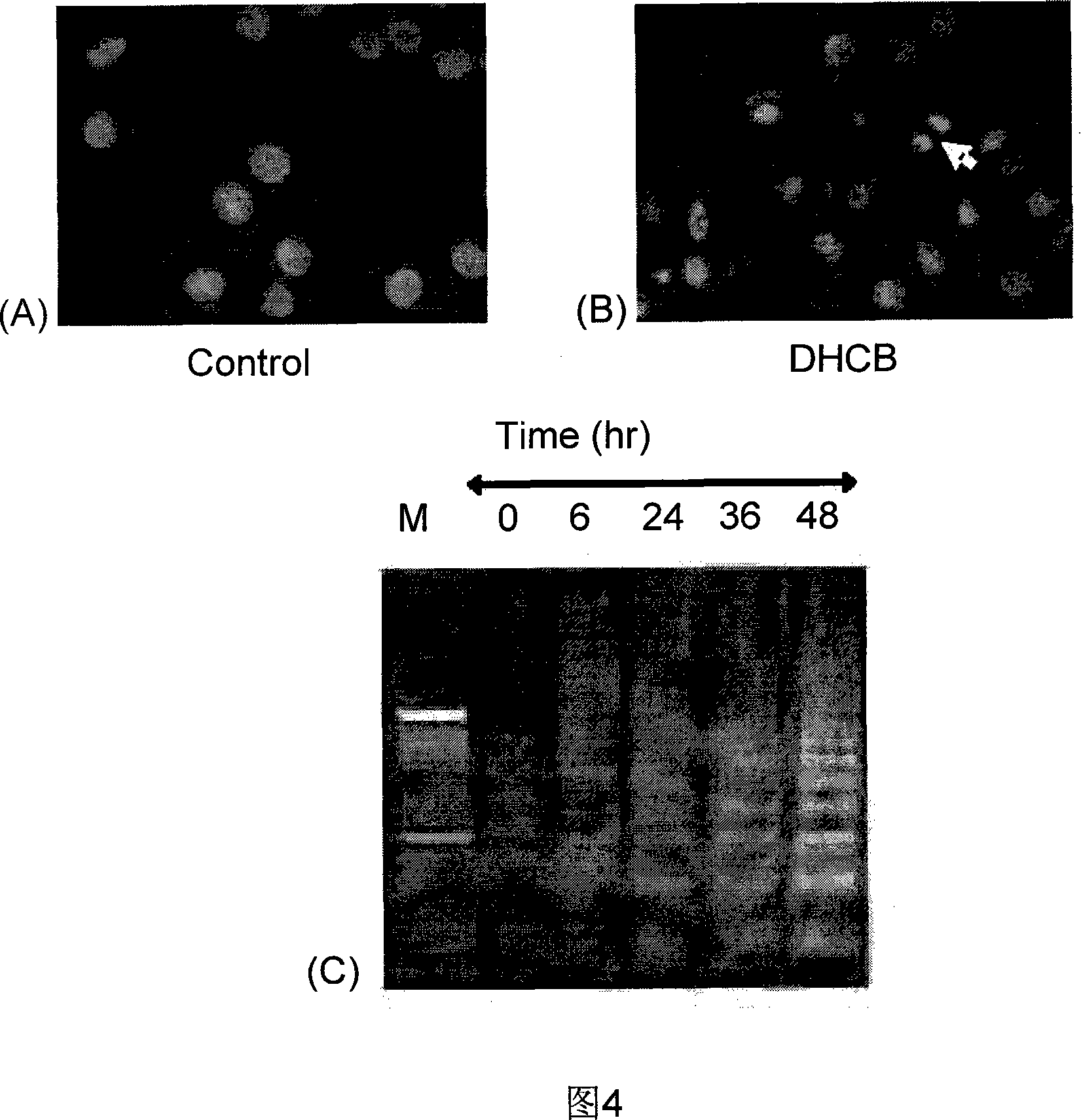
[0039] Example 3: 23,24-Dihydrocucurbitacin B inhibits the apoptosis-related mechanism of breast cancer Bcap37 proliferation
[0040] (1) DNA LADDER experiment: After some cells are treated with drugs, the DNA will be cut by nucleases at the junction of nucleosomes, resulting in DNA fragments of 180-200bp or integer multiples in size, which appear regular in agarose gel electrophoresis This is the most typical feature of apoptosis, and it is also an important sign to distinguish apoptosis from necrosis. The whole experimental process was carried out according to the operation steps of the DNA apoptosis band extraction kit (Biovision). Observe the experimental results under the light.
[0041] The results ( FIG. 4C ) indicated that 3.6 μM 23,24-dihydrocucurbitacin B could significantly induce the apoptosis of breast cancer Bcap37 at 24h, 36h and 48h and produce characteristic DNA ladder-like bands.
[0042] (2) Apoptotic morphology experiment: after breast cancer Bcap37 was co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com