Circular DNA molecule having a conditional origin of replication, process for their preparation and their use in gene therapy
An origin of replication and conditional technology, applied in the direction of recombinant DNA technology, microorganism-based methods, botanical equipment and methods, etc., to achieve the effect of improving cell penetration or distribution ability, and good bioavailability in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0182] Example 1: Construction of XAC-1pir and pir116 host strains by homologous recombination
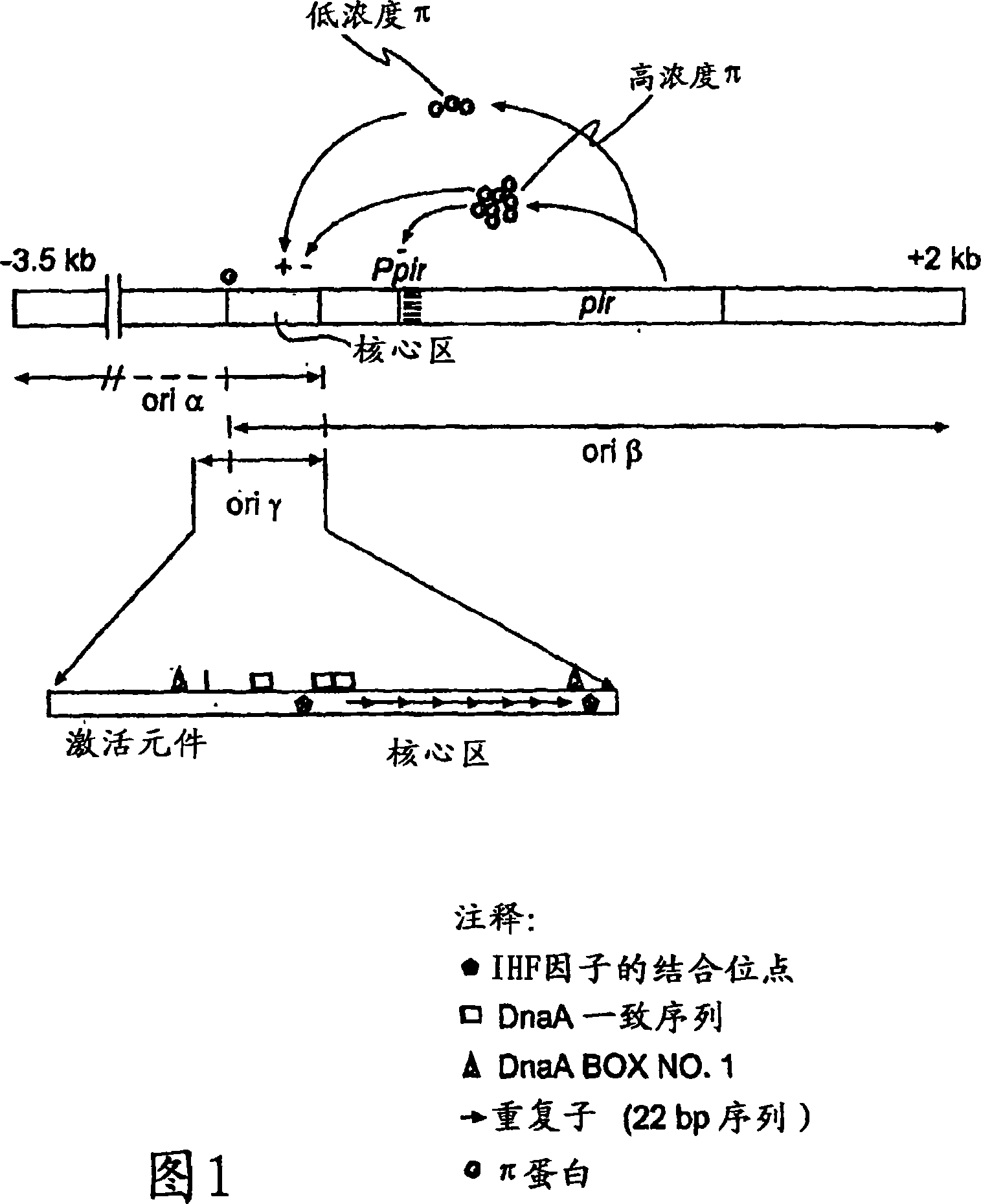
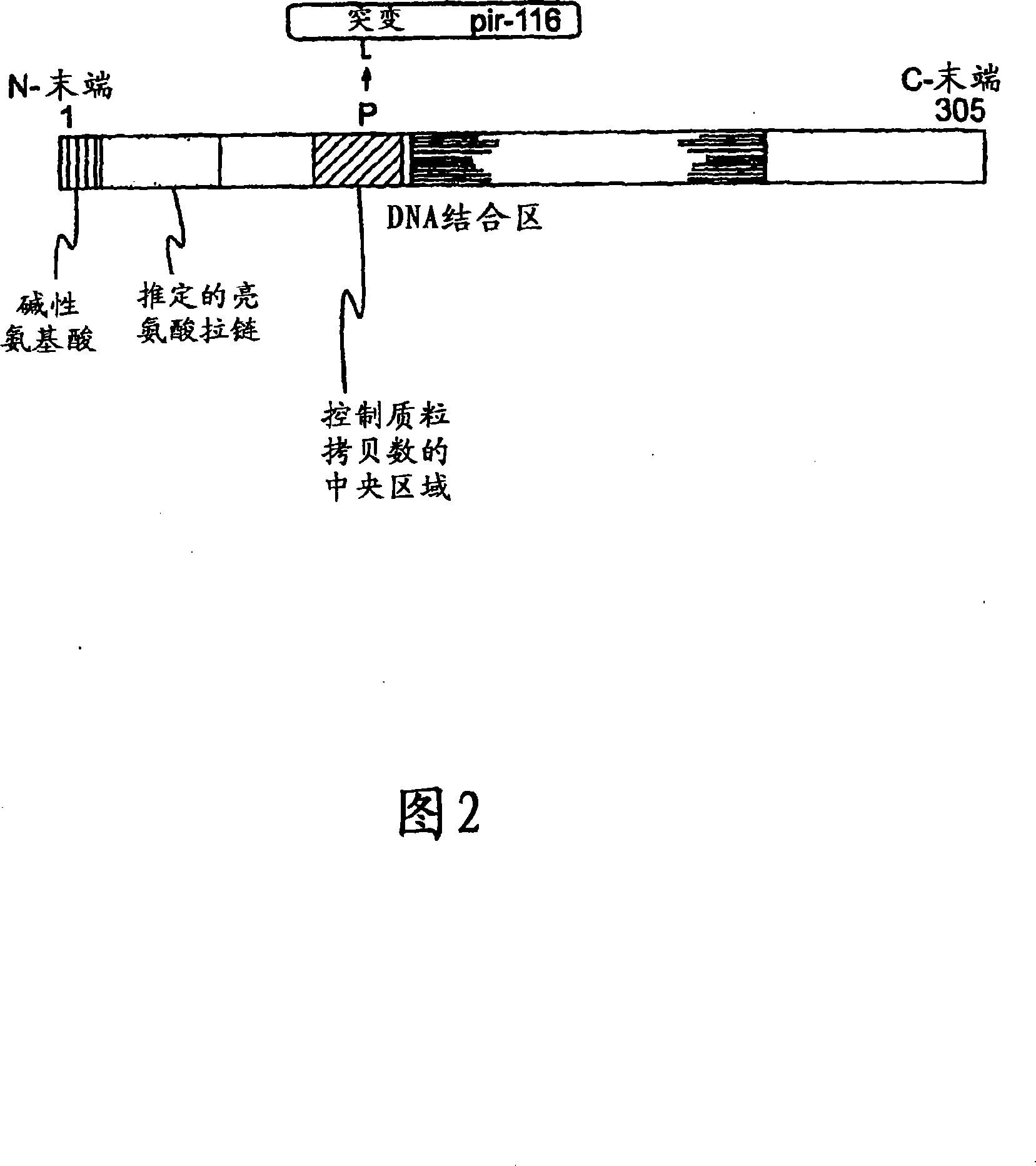
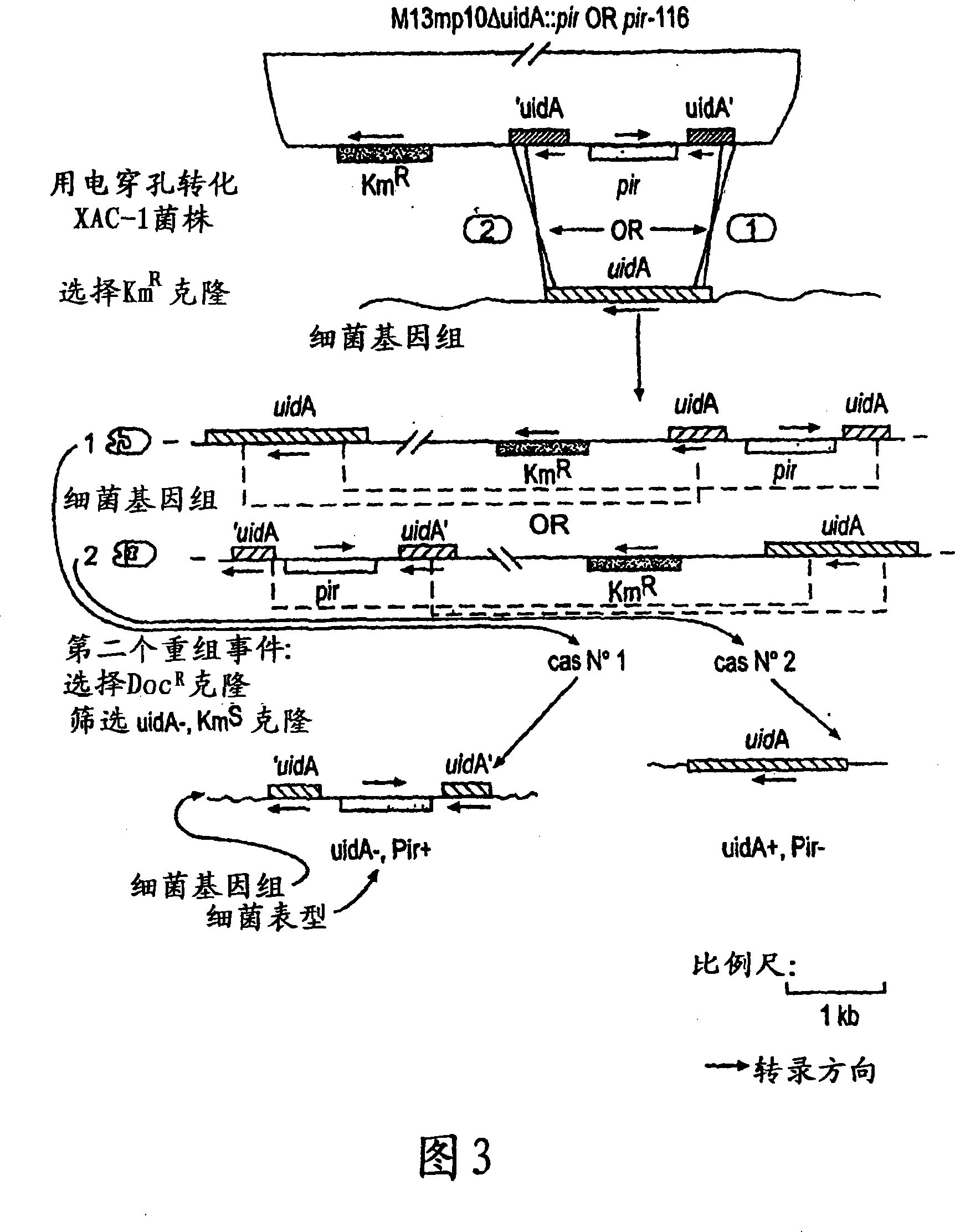
[0183] The strain used was E. coli strain XAC-1 (Normanly et al., 1980). The argE gene of this strain advantageously includes a glutamine-53 (CAG) to amber codon (TAG) mutation (Meinnel et al., 1992). The argE gene belongs to the argECBH divergent operon and encodes the arginine biosynthesis enzyme N-acetylornithinase. Therefore XAC-1 cannot synthesize arginine and thus cannot grow in minimal medium. This auxotrophy will be relieved if the strain contains a plasmid that allows expression of the suppressor tRNA. Bacteria carrying this plasmid can thus be selected for by culturing in minimal medium. In order to allow replication of the R6K-derived plasmid, it was necessary to introduce the pir gene into the XAC-1 genome by homologous recombination. The pir gene (wild type or mutated) was introduced into the uidA locus by exchange between the wild type uidA gene and a copy inter...
Embodiment 2
[0203] Example 2: Construction of a plasmid vector from R6X carrying the selectable marker sup Phe
[0204] A vector (pKL2666) containing oriγ from R6K and a kanamycin resistance gene was constructed. The observation of pXL2666 multimers in strain BW19610(pir116)5 (Metcalf et al., 1993) led us to investigate the influence of the cer fragment of ColE1 on this phenomenon. We then introduced the phenylalanine suppressor tRNA (sup Phe) expression cassette into the vector oriγ-Km R -cer (pXL2730). This vector, pXL2760, serves as the basis for constructing a vector that can be used in gene therapy.
[0205] 1) Construction and analysis of vectors containing R6K oriγ and kanamycin resistance genes
[0206] a) construct
[0207] In the first constructed plasmid pXL2666, the kanamycin resistance gene came from pUC4K (Vieira and Messing; 1982), and the replication origin contained in the 417bp EcoRI-BamHI fragment came from the suicide vector pUT-T7pol (Herrero et al., 1990) (Fi...
Embodiment 3
[0217] Example 3: Confirmation that plasmids can be applied to gene therapy by transfecting mouse fibroblasts
[0218] 1) Construction of reporter vector pXL2774
[0219] To test the effectiveness of this system for generating plasmid DNA for gene therapy, we introduced a reporter gene that can be used in eukaryotic cells into pXL2760. We used the gene luc encoding Photinuspyralis luciferase because bioluminescence measurements are very sensitive, linear over a large range, and background noise due to endogenous activity in eukaryotic cells is very low. The Luc gene is controlled using the promoter-enhancer sequence of the human cytomegalovirus early gene (CMV promoter) allowing high level expression. At the 3' end of luc there is an untranslated region derived from the virus SV40 containing a polyadenylation signal (poly(A)+). After intermediate cloning to increase the number of available restriction sites, the "CMV promoter-luc-poly(A)+" cassette was introduced into th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com