Technique for preparing high quality boric acid from salt lake type boron ore by one-step method
A high-quality and technological technology, applied in the direction of boron oxide compounds, can solve the problems of non-compliance with the quality requirements of boric acid products, low grade and yield, secondary pollution of products, etc., to eliminate secondary pollution, simple operation, boron magnesium Complete separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
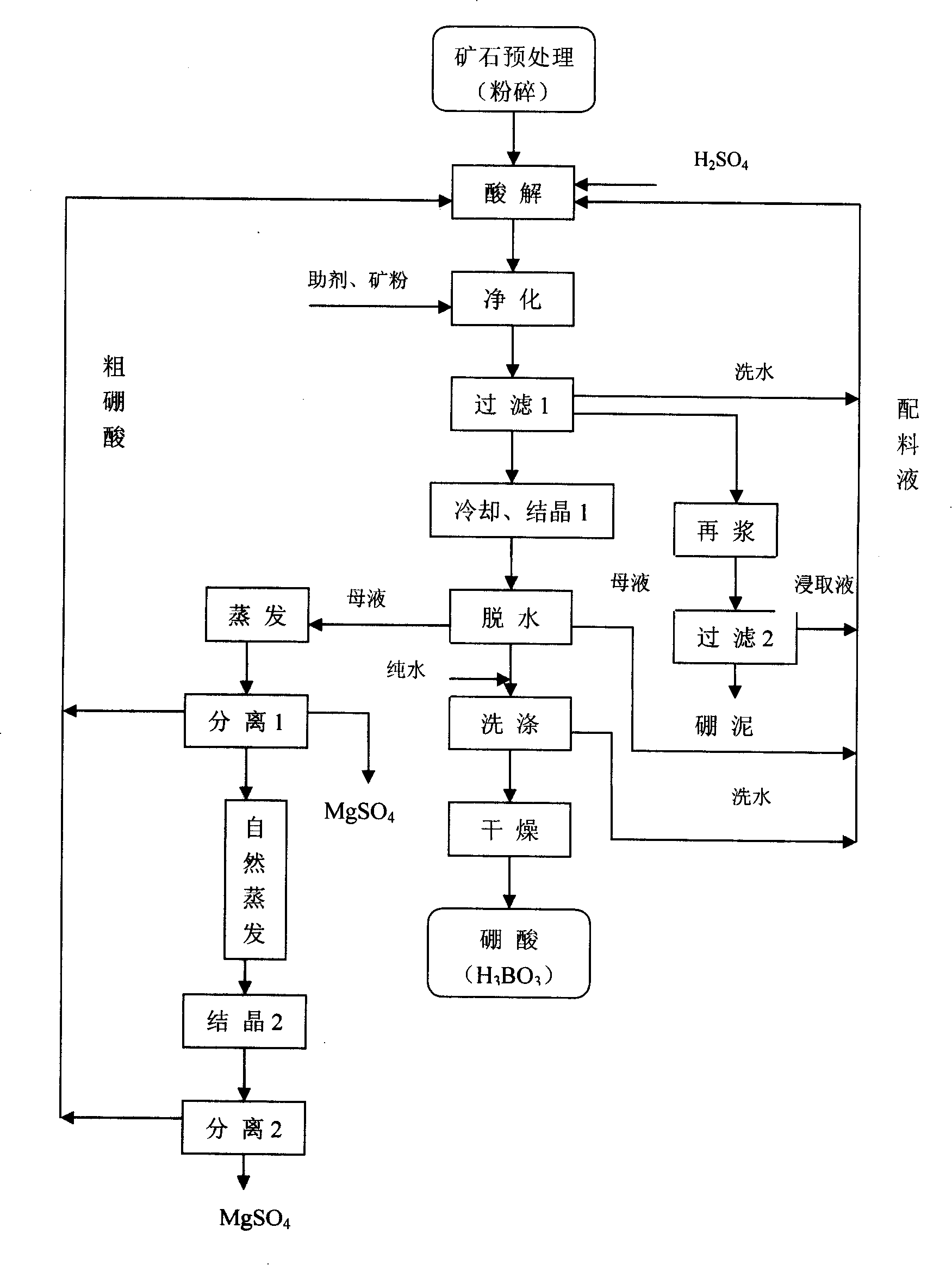
[0036] Embodiment 1: as figure 1 In the process flow chart shown, after the ore is crushed and pretreated, it is acidified with sulfuric acid, and sodium chlorate is added as an iron-resistant solvent in the later stage of the reaction, so that part of the iron ions flow into the solid phase. The chemical reaction formula is ClO 3 - +Fe 2+ +6H + =CL - +Fe 3+ +3H 2 O, Fe 3+ +3H 2 O=Fe(OH) 3 ↓+3H + Afterwards, add oxidant hydrogen peroxide, heavy metal precipitant sodium sulfide or boiler ash to make iron, aluminum and heavy metal ions generate precipitation, or be adsorbed and filtered out. Adding additives in this process can precipitate and filter out. The chemical reaction formula For: 2Fe 2+ +H 2 o 2 +2H + =2Fe 3+ +2H 2 O, Fe 3+ +3H 2 O=Fe(OH) 3 ↓+3H + 、Al 3+ +3H 2 O=Al(OH) 3 ↓+3H + , Mn 2+ +Na 2 S=MnS↓+2Na + , Pb 2+ +Na 2 S=PbS↓+2Na + 、2As 3+ +3Na 2 S=As 2 S 3 ↓+6Na + ; In the filtration 1 process, the boron mud is leached and re-slurried,...
Embodiment 2
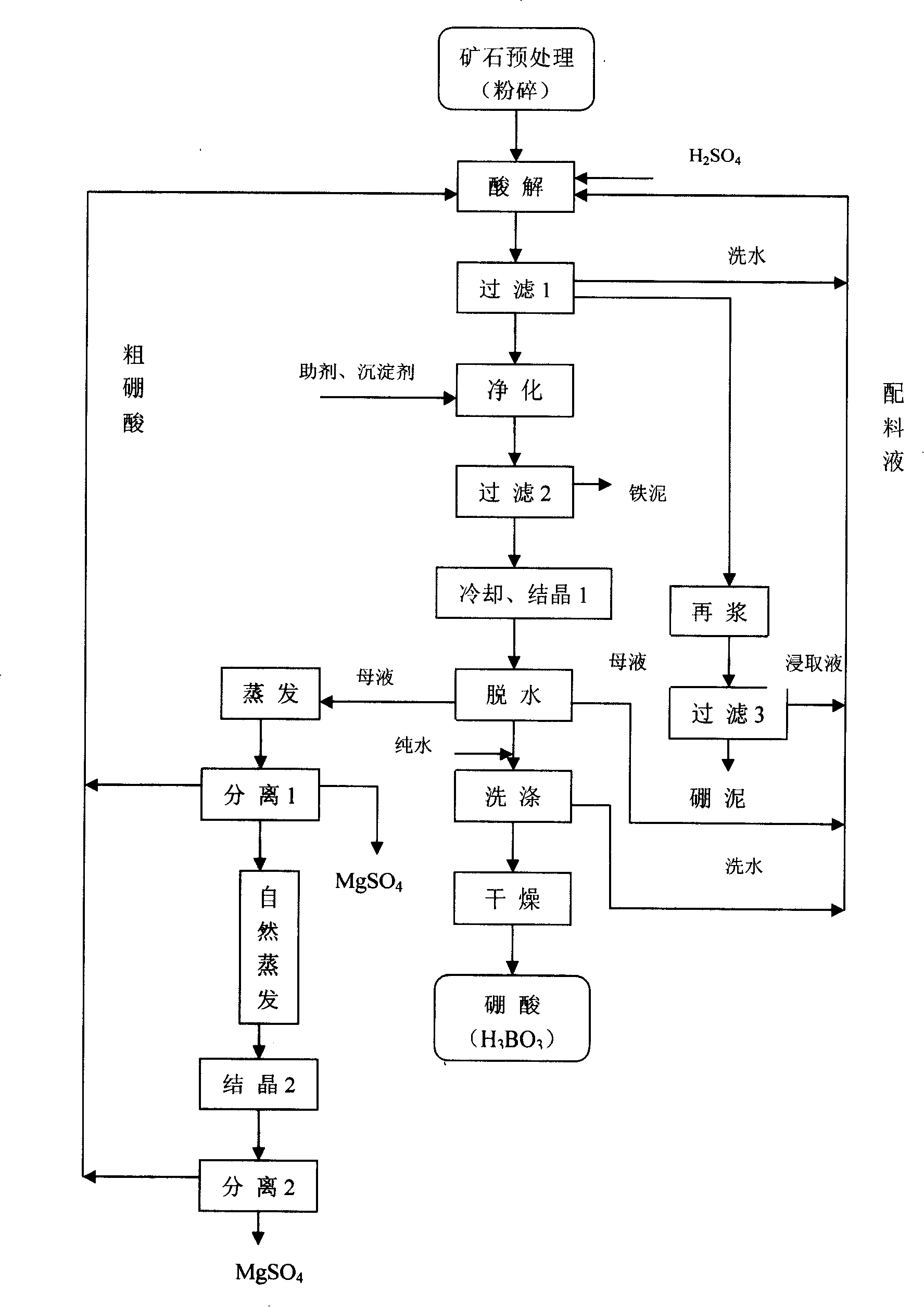
[0041] Embodiment 2: as figure 2 As shown in the process flow diagram, the present invention is to increase oxidation, vulcanization or boiler furnace ash adsorption and impurity removal process between filtration and cooling crystallization 1 process, and before cooling and crystallization after oxidation, vulcanization or boiler furnace ash adsorption and impurity removal process, The mother liquor was filtered again 3, and the filter residue (iron slime) obtained after the filtration was removed, and the filtrate was cooled and crystallized 1; the others were the same as in Example 1.
[0042] In the oxidation and impurity removal process, the present invention adds boron ore powder as a pH regulator, and any one of ammonia water, calcium carbonate powder, sodium hydroxide, and calcium hydroxide solution can also be used as a pH regulator;
[0043] In the oxidative impurity removal process, in addition to hydrogen peroxide, sodium hypochlorite can also be used as an oxidan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com