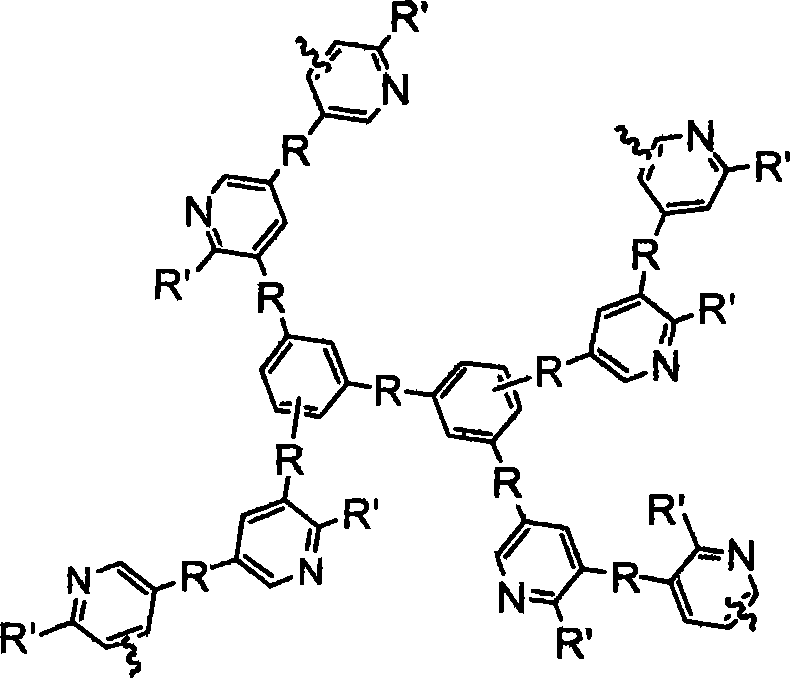
Hyperbranched polyaromatic hydrocarbon containing pyridine rings and method for producing the same
A technology of pyridine ring and polyaromatic hydrocarbon is applied in the field of hyperbranched polymers to achieve the effects of good wave absorption performance, good electron transmission performance and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: Biphenyls
[0038] Synthesis principle equation:
[0039]
[0040] (1) Synthesis of 1,4-diol base biphenyl (2):
[0041] 1,4-Dibromobiphenyl (5g, 16.02mmol), Pd(Ph 3 P) 2 Cl 2 (11.2mg, 0.016mmol), cuprous iodide (3mg, 0.016mmol), triphenylphosphine (10mg, 0.016mmol) were added to a 100mL (19#) single-necked round bottom flask, and under nitrogen protection, toluene (20mL ), triethylamine (20mL), 2-methyl-3-butyn-2-ol (2.4mL, 24.6mmol), the temperature was maintained between 90-100°C, and the reaction was carried out under nitrogen atmosphere for 36h. The product was filtered and washed with anhydrous ether. The solvent was distilled off under atmospheric pressure, and purified by column chromatography (developer: petroleum ether / ethyl acetate=3 / 1) to obtain 4.0 g of yellow viscous liquid with a yield of 80%.
[0042] (2) Synthesis of 1,4-diynylbiphenyl (3):
[0043] Add 1,4-diol-based biphenyl (2) (3g, 12.2mmol), sodium hydroxide (0.48g, 12.2mmol...
Embodiment 1-1
[0044] Example 1-1: Synthesis of biphenyl conjugated polymer P1
[0045] Put 1,4-(diynyl)biphenyldiyne (50mg, 0.25mmol) into a thoroughly dried test tube, dissolve valeronitrile (26 μL, 0.25mmol) in (0.2mL) toluene, and add it to the test tube; Add dissolved CpCo(CO) 2 (4.5mg, 0.025mmol) of toluene (1.0mL); the mixture was stirred under the irradiation of a 300w Hg lamp, the lamp was 25cm away from the center of the test tube, and the irradiation was continued at 65°C for 10h; a small amount of methanol was added to stop the polymerization reaction. Stir well to precipitate the polymer, pour the polymer solution into 100 mL of methanol through a cotton filter, collect a light yellow powder, and dry it to constant weight under vacuum. Yield: 36 mg (77%) 13 C NMR analysis of its structure showed that it was the target product.
Embodiment 1-2
[0046] Example 1-2: Synthesis of biphenyl conjugated polymer P2
[0047] 1,4-(diynyl)biphenyldiyne (50mg, 0.25mmol) was put into a thoroughly dried test tube, valeronitrile (12 μL, 1.24mmol) was dissolved in (0.2mL) toluene, and added to the test tube; Add dissolved CpCo(CO) 2 (4.5mg, 0.025mmol) of toluene (1.0mL); the mixture was stirred under the irradiation of a 300w Hg lamp, the lamp was 25cm away from the center of the test tube, and the irradiation was continued at 65°C for 10h; a small amount of methanol was added to stop the polymerization reaction. Stir well to precipitate the polymer, pour the polymer solution into 100 mL of methanol through a cotton filter, collect a light yellow powder, and dry it to constant weight under vacuum. Yield: 36 mg (75%) 13 C NMR analysis of its structure showed that it was the target product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com