Aspergillus flavus toxin immuno-affinity column preparation method
A technology of aflatoxin and immunoaffinity, which is applied in the field of preparation of immunoaffinity columns, can solve the problems of increasing non-specific adsorption, cyanogen bromide is highly toxic, and cross-linked antibodies are uneconomical, so as to avoid non-specific adsorption, good active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment one: the specific steps of a preferred embodiment of the present invention are:
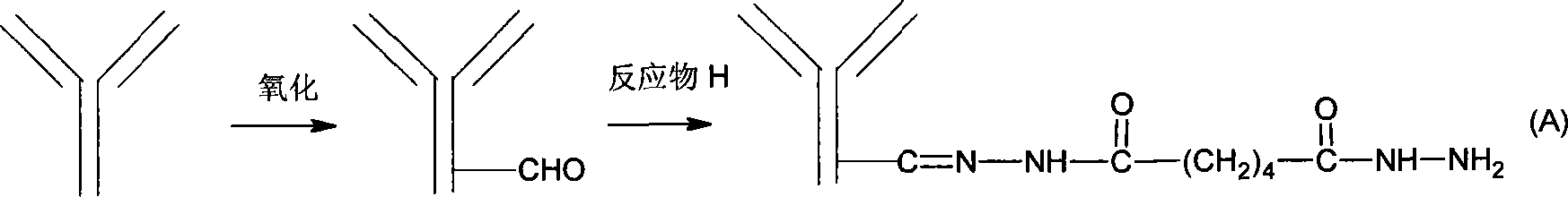
[0031] 1) Preparation of hydrazide antibody
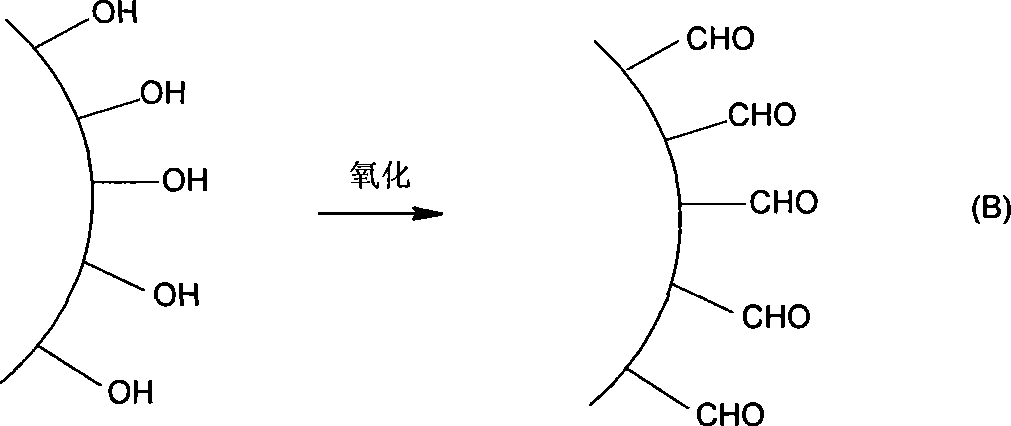
[0032] Precipitate the ascites (aflatoxin antibody) with ammonium sulfate, centrifuge, remove the supernatant, dissolve the precipitate with distilled water to make the antibody concentration greater than 25mg / ml, and dialyze against pH 5.8 0.2M sodium phosphate buffer. Add one-tenth volume of 20-40 mg / ml sodium periodate aqueous solution to the antibody solution, stir and react at room temperature in the dark for 1 hour, and separate the antibody through Sephadex G25. Add excess adipate dihydrazide aqueous solution (pH 5.8) to the above-mentioned sodium periodate oxidized antibody (add 0.5-2 mg adipate dihydrazide per mg of antibody), react overnight at 4°C, pH 5.8 for 0.2M sodium phosphate Dialysis. 2) Preparation of aldylated solid phase matrix (Sepharose 4B)
[0033] Take an appropriate amount of Sepharose 4B and drain, weigh ...
Embodiment 2
[0042] Embodiment 2 Immunoaffinity Column Fluorophotometry Determination of Aflatoxins
[0043] In the blank sample (Canadian wheat) without AFT, add 1, 5, 20, 50ppb aflatoxin mixed standard (AFB 1 , AFB 2 , AFG 1 , AFG 2 ), the sample extract was purified with the affinity column prepared in Example 1, and the concentration was measured with a fluorophotometer. The specific operation method refers to the national standard GB / T 18979-2003. The results are shown in Table 1 as follows:
[0044]Table 1 adopts the immunoaffinity column prepared by the present invention and the result of measuring aflatoxin by fluorescence photometry
[0045]
[0046] As can be seen from the above data, in the blank sample Canadian wheat, 4 levels of concentration addition recovery experiments are carried out to AFT, the average recovery rate is 82.7~99.3%, and the coefficient of variation is 5.3~9.3%, which shows that the aflatoxin prepared by the present invention The immunoaffinity colu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com